Highlights
The Glycemia Reduction Approaches in Diabetes: A Comparative Effectiveness Study (GRADE) has provided a landmark analysis of how different glucose-lowering medications influence the complex interplay between the alpha and beta cells of the pancreas. Key takeaways include:
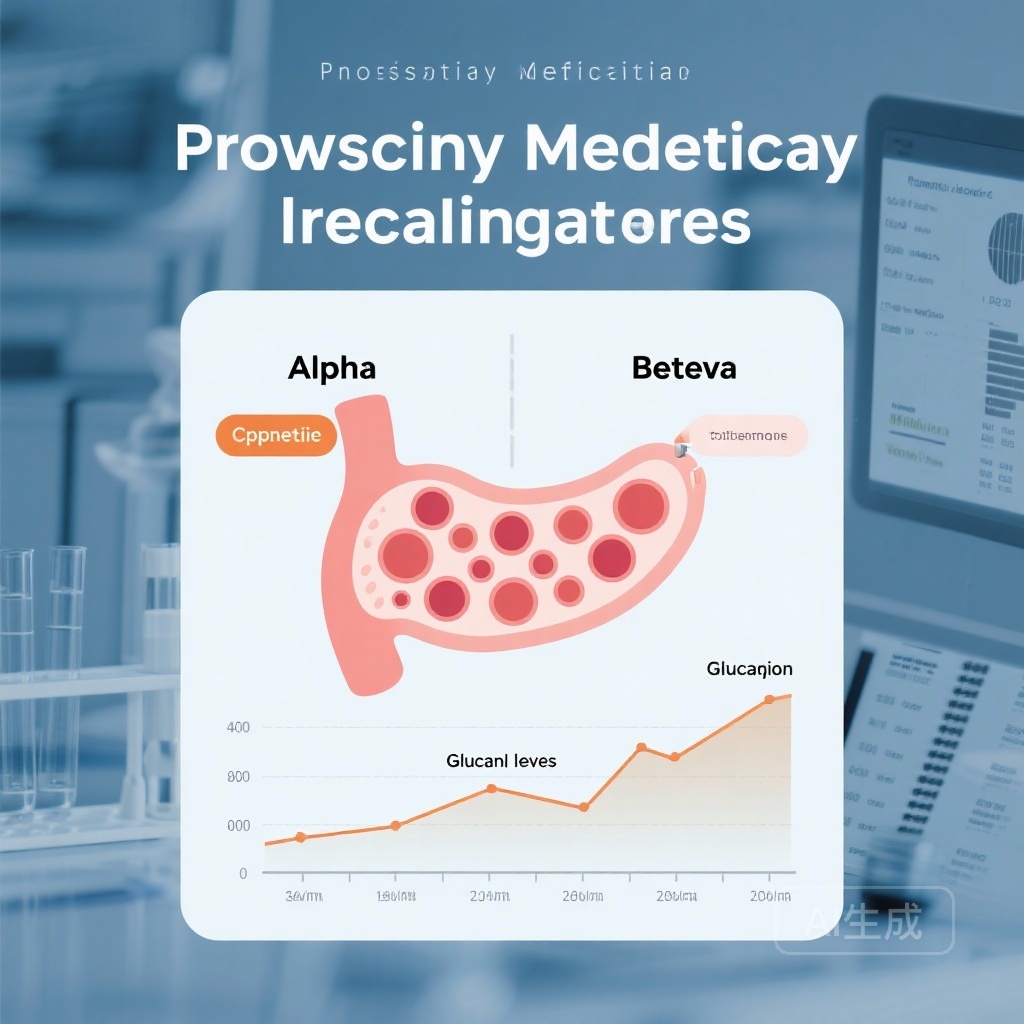
- Baseline fasting glucagon and the glucagon index (GGI) were not associated with the primary metabolic outcome of reaching an HbA1c ≥7.0%.
- In contrast, beta-cell function measures—specifically the C-peptide index (CPI)—were significantly associated with long-term glycemic control.
- A 1-standard deviation (SD) increase in the C-peptide index was linked to a 17% reduction in the risk of glycemic failure.
- While various agents (glargine, glimepiride, liraglutide, and sitagliptin) had different longitudinal effects on glucagon levels, these alpha-cell changes did not independently predict metabolic success or failure.
Background: The Bi-hormonal Hypothesis in Type 2 Diabetes
For decades, the pathophysiology of type 2 diabetes (T2D) has been viewed through the lens of the “bi-hormonal hypothesis.” This model suggests that hyperglycemia is not merely a consequence of insulin deficiency or resistance (beta-cell failure) but is also driven by the inappropriate hypersecretion of glucagon by pancreatic alpha-cells. In a healthy physiological state, glucagon secretion is suppressed following glucose ingestion to prevent excessive hepatic glucose production. In T2D, this suppression is often lost, leading to postprandial hyperglycemia.
Therapeutic agents such as GLP-1 receptor agonists (liraglutide) and DPP-4 inhibitors (sitagliptin) are known to modulate the alpha-cell response. However, the longitudinal impact of these medications on alpha-cell function—and more importantly, whether that impact translates to clinical durability—has remained a subject of intense debate. The GRADE study sought to clarify whether the preservation or restoration of alpha-cell function is a viable clinical target compared to the traditional focus on beta-cell health.
Study Design and Methodology: The GRADE Subset Analysis
The GRADE study was a multicenter, randomized comparative effectiveness trial that enrolled 5,047 participants with T2D who were already receiving metformin. Participants had a baseline HbA1c between 6.8% and 8.5% and were randomized to receive one of four adjunct therapies: insulin glargine U100, the sulfonylurea glimepiride, the GLP-1 receptor agonist liraglutide, or the DPP-4 inhibitor sitagliptin.
In a sophisticated subset analysis of 724 participants, researchers measured fasting glucagon concentrations and the change in glucagon from fasting to 30 minutes following an oral glucose challenge (the glucagon index, or GGI). Simultaneously, they assessed beta-cell function using fasting C-peptide and the C-peptide index (CPI). The primary metabolic outcome was the time to a confirmed HbA1c ≥7.0%. By following these biomarkers longitudinally, the study aimed to determine if changes in alpha-cell function contributed to the durability of the various treatment regimens.
Key Results: Dissecting Alpha and Beta Cell Contributions
The Primacy of the Beta-Cell
The most striking finding from the GRADE analysis was the stark difference in the predictive value of beta-cell versus alpha-cell markers. Baseline measures of beta-cell function (fasting C-peptide and CPI) were significantly associated with the primary metabolic outcome (P = 0.04). Specifically, participants with more robust beta-cell responses at the start of the study were less likely to experience glycemic failure over time.
Longitudinally, the importance of the beta-cell became even clearer. Changes in the C-peptide index were strongly associated with the risk of reaching an HbA1c ≥7.0%. Even after adjusting for alpha-cell function, a 1-SD increase in CPI corresponded to a 17% lower risk of metabolic failure. This suggests that the ability of a medication to maintain or enhance insulin secretion (as measured by C-peptide) is the dominant factor in long-term glucose management.
The Paradox of Alpha-Cell Function
Contrary to the expectations of the bi-hormonal model, alpha-cell function showed no significant association with glycemic failure. Neither the baseline fasting glucagon nor the GGI predicted which patients would eventually exceed an HbA1c of 7.0% (P = 0.55). Furthermore, treatment-associated changes in glucagon levels over time did not correlate with the primary metabolic outcome.
While the four medications exhibited different longitudinal patterns regarding glucagon levels—reflecting their different mechanisms of action—these differences did not translate into a differential clinical benefit regarding glycemic durability. For example, while liraglutide and sitagliptin are known to affect the incretin axis and suppress glucagon, this suppression did not appear to be the primary driver of their efficacy in this cohort compared to agents like glargine or glimepiride.
Expert Commentary: Shifting the Clinical Focus
The results of the GRADE study provide a necessary reality check for clinical endocrinology. While the alpha-cell remains a fascinating component of islet biology, its clinical relevance as a therapeutic target for preventing secondary failure of metformin-based therapy appears limited. The data reinforces the “beta-cell-centric” view of T2D progression.
Mechanistically, this suggests that the glucose-lowering effects of incretin-based therapies may rely more heavily on their insulinotropic effects rather than their glucagonostatic effects. From a health policy and clinical practice perspective, these findings suggest that when choosing a second-line agent after metformin, clinicians should prioritize medications that demonstrate the greatest potential for preserving beta-cell function or providing exogenous insulin replacement when endogenous capacity wanes.
However, some limitations must be noted. The study measured glucagon at 30 minutes post-glucose challenge, which may not capture the full dynamic range of alpha-cell suppression. Additionally, the study focused on the transition to an HbA1c of 7.0%; it is possible that alpha-cell function plays a more significant role in more advanced stages of the disease or in the prevention of hypoglycemia, which was not the primary focus of this specific analysis.
Conclusion: Practical Implications for Diabetes Care
The GRADE study concludes that the longitudinal effects of various glucose-lowering medications on alpha-cell function do not relate to the worsening of glycemia. Consequently, for the management of type 2 diabetes, the selection of pharmacotherapy should remain focused on the impact on beta-cell function and insulin sensitivity.
As we move toward more personalized medicine in diabetes care, the C-peptide index serves as a valuable biomarker for predicting treatment durability. While researchers will continue to explore the nuances of islet cell crosstalk, the clinician’s primary mission remains the preservation of the beta-cell to ensure long-term metabolic stability for patients with T2D.
Funding and ClinicalTrials.gov
The GRADE study was supported by grants from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) of the National Institutes of Health (NIH). Additional support was provided by the National Center for Advancing Translational Sciences (NCATS). ClinicalTrials.gov Identifier: NCT01794143.
References
Kahn SE, Tripputi M, Lachin JM, et al.; GRADE Research Group. Differential Longitudinal Effects of Glucose-Lowering Medications on Glucagon and C-peptide Responses in the Glycemia Reduction Approaches in Diabetes: A Comparative Effectiveness Study (GRADE). Diabetes Care. 2026 Feb 1;49(2):325-334. doi: 10.2337/dc25-2186. PMID: 41432725.
Nathan DM, Buse JB, Kahn SE, et al.; GRADE Study Research Group. Rationale and design of the Glycemia Reduction Approaches in Diabetes: A Comparative Effectiveness Study (GRADE). Diabetes Care. 2013;36(8):2254-2261. doi:10.2337/dc13-0356.
Ungar RH, Cherrington AD. Glucagonocentric restructuring of diabetes: a pathophysiologic and therapeutic makeover. J Clin Invest. 2012;122(1):4-12. doi:10.1172/JCI60016.