Highlights
- Urolithin A (UA) supplementation improved muscle endurance and isometric strength in resistance-trained male athletes.
- UA led to reductions in key inflammatory and oxidative stress markers after 8 weeks.
- No significant impact was observed on maximal dynamic strength (1RM) or protein catabolism markers compared to placebo.
- UA showed a favorable safety profile with no significant adverse effects.
Study Background and Disease Burden
Sarcopenia, athletic overtraining, and muscle recovery remain major concerns among both clinical and athletic populations. Enhancing muscle performance while mitigating inflammation and oxidative stress is critical for optimal adaptation and injury prevention in resistance-trained individuals. Urolithin A (UA), a gut microbiome-derived metabolite of ellagitannins found in foods like pomegranates and walnuts, has emerged as a potential ergogenic aid due to its role in mitochondrial health, anti-inflammatory pathways, and cellular homeostasis. However, robust clinical data in human athletes are limited. This study addresses the gap by rigorously evaluating UA’s effects on muscle performance, inflammation, oxidative stress, and protein metabolism in resistance-trained male athletes.
Study Design
This was an 8-week, randomized, double-blind, placebo-controlled trial conducted by Zhao et al. Twenty resistance-trained male athletes were enrolled and randomized to receive either 1 g/day of UA or placebo. Key inclusion criteria were regular engagement in resistance training and absence of underlying health conditions. Both groups were matched for baseline characteristics and dietary intake.
Interventions:
– UA group: 1 g Urolithin A orally, daily
– Placebo group: Matched placebo capsule
Primary endpoints:
– Muscle strength (1-repetition maximum [1RM] for bench press and squat)
– Muscle endurance (maximum voluntary isometric contraction [MVIC], repetitions to failure [RTF])
Secondary endpoints:
– Inflammatory markers (C-reactive protein [CRP], interleukin-6 [IL-6])
– Oxidative stress (superoxide dismutase [SOD])
– Protein metabolism (urinary 3-methylhistidine [3-MH])
Measurements were obtained at baseline and after 8 weeks, including fasting venous blood, morning urine, and comprehensive dietary analysis to control for confounding nutritional variables.
Key Findings
Muscle Performance:
– UA group showed non-significant improvements in 1RM bench press (Δ = 3.00 ± 0.17 kg, p = 0.051) and squat (Δ = 1.35 ± 2.73 kg, p = 0.499) compared to baseline.
– Significant enhancements were observed in MVIC (Δ = 36.10 ± 0.62 NM, p = 0.000) and RTF (Δ = 2.00 ± 0.56, p = 0.001) in the UA group.
– Compared to placebo, UA supplementation led to significant improvements in MVIC (Δ = 43.50 ± 0.77 NM, p = 0.048) and RTF (Δ = 2.00 ± 1.22, p = 0.011), but not in 1RM measures.
Inflammatory and Oxidative Stress Markers:
– UA group exhibited a significant reduction in CRP compared to placebo (Δ = -0.79 ± 0.38 mg/L, p = 0.032), indicating a systemic anti-inflammatory effect.
– No significant changes in IL-6 were observed (Δ = -1.75 ± 0.45 pg/mL, p = 0.215 vs placebo).
– UA group showed a significant decrease in SOD compared to placebo (Δ = -4.32 ± 0.90 U/mL, p = 0.041), suggesting reduced oxidative stress burden.
Protein Metabolism:
– UA group demonstrated a significant decrease in urinary 3-MH from baseline (Δ = -2.38 ± 1.96 μmol/L, p = 0.049), but the difference was not significant relative to placebo.
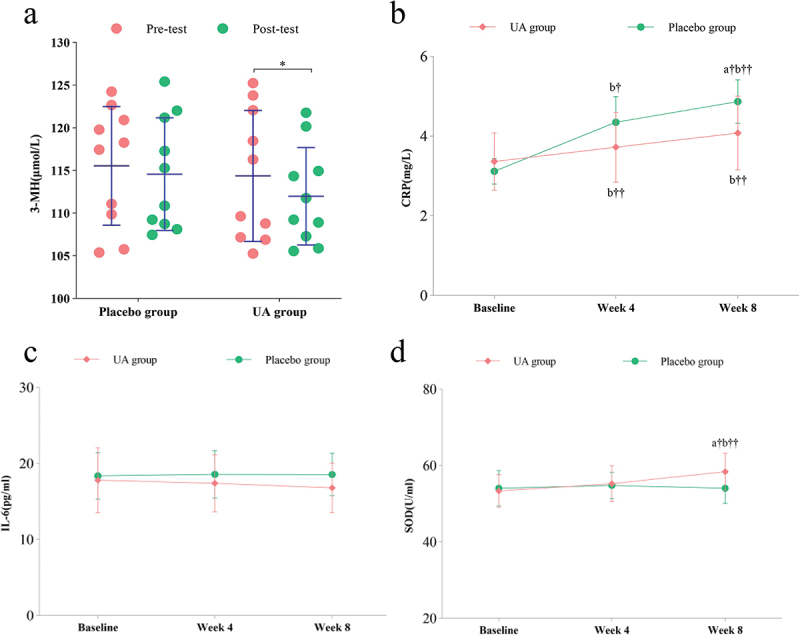
6 weeks of supplementation, the changes in urinary and blood indicators were assessed in both the placebo and UA groups (a)3-mh. (b)CRP (c)IL-6. (d)SOD.
Dietary Control and Safety:
– No significant differences in energy or macronutrient intake were observed between groups or over time, minimizing dietary confounding.
– UA was well tolerated, with no reported adverse events.
Expert Commentary and Biological Plausibility
This study provides preliminary evidence that UA supplementation can enhance certain facets of muscular performance, particularly isometric strength and endurance, in trained athletes—a demographic where further gains are typically difficult to achieve. The observed reduction in CRP and SOD supports a mechanistic link between UA and attenuation of exercise-induced inflammation and oxidative stress, which are implicated in muscle fatigue and delayed recovery. The lack of significant change in dynamic maximal strength (1RM) may reflect the short intervention period or ceiling effects in well-trained individuals. The reduction in 3-MH, a biomarker of muscle protein breakdown, suggests a potential anti-catabolic effect, though further studies with larger samples are needed.
Limitations include the small sample size, short duration, and restriction to male athletes, which may limit generalizability. Additionally, the lack of change in IL-6 and the paradoxical increase in CRP at baseline within the UA group warrant further exploration. Nonetheless, the trial’s double-blind, placebo-controlled design and dietary control strengthen the validity of findings.
Conclusion
Eight weeks of Urolithin A supplementation at 1 g/day in resistance-trained male athletes significantly improved muscle endurance and isometric strength, with concurrent reductions in markers of inflammation and oxidative stress, and a trend toward reduced muscle protein breakdown. These findings support UA as a promising adjunct for athletic performance and recovery, though larger, longer-term trials across diverse populations are needed to confirm efficacy and safety.
References
Zhao H, Zhu H, Yun H, Liu J, Song G, Teng J, Zou D, Lu N, Liu C. Assessment of Urolithin A effects on muscle endurance, strength, inflammation, oxidative stress, and protein metabolism in male athletes with resistance training: an 8-week randomized, double-blind, placebo-controlled study. J Int Soc Sports Nutr. 2024 Dec;21(1):2419388. doi: 10.1080/15502783.2024.2419388 IF: 3.9 Q1 . Epub 2024 Nov 2. PMID: 39487653 IF: 3.9 Q1 ; PMCID: PMC11536656 IF: 3.9 Q1 .
Additional relevant literature:
– Andreux PA, et al. The mitophagy activator urolithin A is safe and induces a molecular signature of improved mitochondrial and cellular health in humans. Nat Metab. 2019;1(6):595-603.
– Tomás-Barberán FA, et al. Urolithins, the main human metabolites of ellagitannins: molecular mechanisms and biomedical relevance. Mol Nutr Food Res. 2021;65(1):e1900949.



