Posted inCardiology news
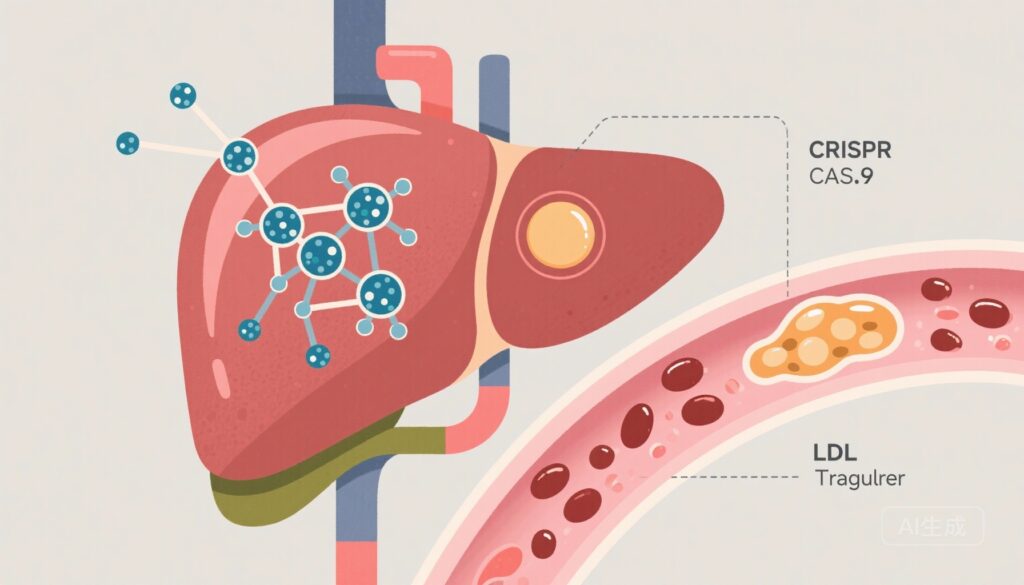
First-in-Human CRISPR-Cas9 Editing of ANGPTL3 Shows Dose‑Dependent Protein Knockdown with Acceptable Short‑Term Safety in Phase 1 Trial
A phase 1, ascending‑dose study of CTX310 (LNP-delivered CRISPR-Cas9 targeting ANGPTL3) in 15 patients produced dose-dependent ANGPTL3 reductions at ≥0.6 mg/kg with few acute safety signals; longer follow‑up and larger trials are required to define efficacy, durability, and long-term risks.