Highlights
- Ruxolitinib provides significantly longer failure-free survival (FFS) and duration of response (DOR) than best available therapy (BAT) in patients with corticosteroid-refractory or dependent chronic graft-versus-host disease (SR/D-cGVHD).
- Over three years, ruxolitinib maintains a favorable benefit-risk profile, with sustained responses and lower rates of severe adverse events compared to BAT.
- Corticosteroid-sparing effects were evident, with more patients able to discontinue steroids on ruxolitinib than on BAT.
Study Background and Disease Burden
Chronic graft-versus-host disease (cGVHD) is a major complication following allogeneic hematopoietic stem cell transplantation (HSCT), affecting up to 50% of recipients and significantly impacting long-term morbidity and mortality. Corticosteroids are the cornerstone of first-line therapy, but 50–60% of patients become refractory or dependent, necessitating effective second-line options. Until recently, there were limited evidence-based alternatives, and prolonged steroid use is associated with considerable toxicity and impaired quality of life. The unmet need for durable, safe, and steroid-sparing therapies in SR/D-cGVHD prompted the evaluation of targeted agents such as ruxolitinib, a selective JAK1/2 inhibitor.
Study Design
REACH3 (NCT03112603) was a global, randomized, multicenter, open-label phase III trial enrolling patients aged ≥12 years with moderate-to-severe SR/D-cGVHD. Participants (N=329) were randomized 1:1 to receive either ruxolitinib (10 mg twice daily) or investigator-selected best available therapy (BAT), which included various immunosuppressants per local standard. The primary efficacy period was 24 weeks, after which patients could continue their assigned therapy, or those on BAT could cross over to ruxolitinib from week 24 onwards. Long-term follow-up extended to 156 weeks (three years) assessing efficacy, safety, corticosteroid use, and survival outcomes.
Key endpoints included:
– Failure-free survival (FFS): time to relapse, non-relapse mortality (NRM), or need for new systemic therapy
– Duration of response (DOR)
– Overall survival (OS)
– Adverse events (AEs), focusing on grade ≥3 and serious events
– Corticosteroid tapering and discontinuation rates
Key Findings
Efficacy Outcomes
Ruxolitinib demonstrated clear and sustained clinical advantages over BAT:
– Median FFS was markedly longer with ruxolitinib (38.4 months) versus BAT (5.7 months), with a hazard ratio (HR) of 0.36 (95% CI, 0.27 to 0.49), indicating a 64% reduction in the risk of failure events. At 36 months, 56.5% of ruxolitinib-treated patients were failure-free, compared to 18.2% on BAT.
FIG 1.
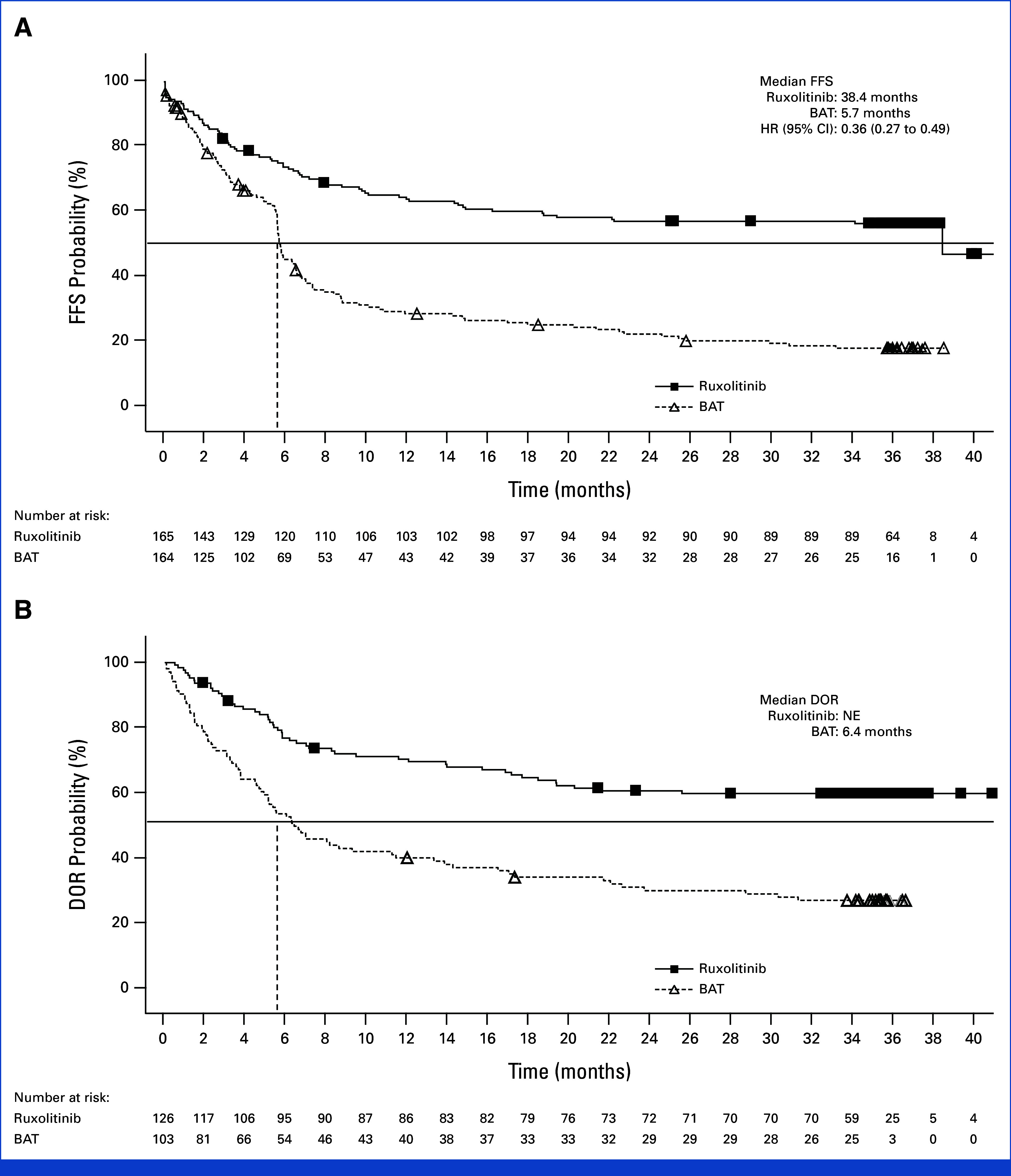
Kaplan-Meier analysis of (A) FFS and (B) DOR in the full analysis set. FFS is a composite end point that includes relapse/recurrence of underlying disease or death due to underlying disease, nonrelapse mortality, or addition/initiation of another systemic therapy for cGVHD. DOR is defined as the first response until progression, death, or the date of additional systemic therapies. BAT, best available therapy; cGVHD, chronic graft-versus-host disease; DOR; duration of response; FFS, failure-free survival; HR, hazard ratio; NE, not estimable (not reached).
– Duration of response (DOR) was not reached for ruxolitinib (i.e., most responders remained in remission at data cut-off), while it was 6.4 months (95% CI, 4.9 to 11.4) in the BAT arm. The probability of maintaining a response at 36 months was 59.6% for ruxolitinib, versus 26.7% for BAT.
– Among patients who discontinued ruxolitinib after 24 weeks, only 14% (3 of 21) experienced a cGVHD recurrence, suggesting the potential for durable remission.
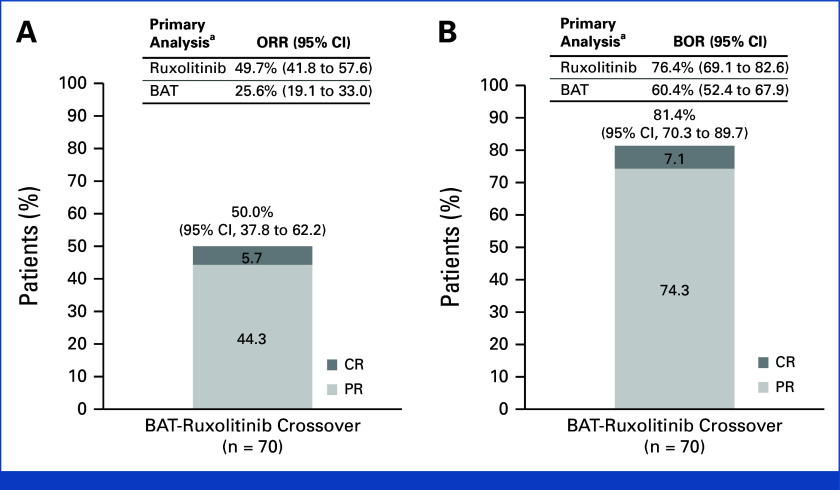
(A) ORR at week 24 and (B) BOR up to week 24 of the crossover period in the crossover population and in the primary analysis population at week 24 of the primary treatment period for REACH3. aORR was the primary end point and BOR was a key secondary end point in the primary analysis of the REACH3 at week 24.2 BAT, best available therapy; BOR, best overall response; CR, complete response; ORR, overall response rate; PR, partial response.
– Median overall survival (OS) was not reached in either arm, with comparable death rates (HR, 0.85; 95% CI, 0.54 to 1.33). Non-relapse mortality and malignancy relapse/recurrence rates were low and similar across groups at 36 months.
– Corticosteroid dose reduction was greater with ruxolitinib: 55.3% of patients fully discontinued steroids versus 36.4% in BAT.
– The sustained benefit of ruxolitinib was consistent across subgroups defined by organ involvement, including high-risk categories such as lung and liver cGVHD.
Safety Profile
– Serious and grade ≥3 adverse events, adjusted for exposure, were less frequent with ruxolitinib than BAT (grade ≥3 AEs: 94.1 vs 120.9 per 100 patient-treatment years; serious AEs: 43.3 vs 63.9 per 100 PTY).
– The most common adverse event with ruxolitinib was anemia, followed by creatinine increase, both manageable with dose adjustments or supportive care. Pneumonia was the leading cause of discontinuation, though rates were not higher than BAT.
– There was no significant increase in infection-related complications or secondary malignancies with ruxolitinib compared to BAT.
Expert Commentary
The three-year final analysis of REACH3 provides compelling evidence for ruxolitinib as the standard of care for SR/D-cGVHD following steroid failure. Its benefit in extending FFS and DOR, combined with a manageable safety profile and the ability to reduce or discontinue corticosteroids, directly addresses the limitations of historical second-line therapies. These results are consistent with guideline endorsements by the European Society for Blood and Marrow Transplantation and regulatory approvals in the US and Europe.
A notable strength of REACH3 is the long-term follow-up, which captures the chronicity and fluctuating nature of cGVHD, and the allowance for crossover, which reflects real-world therapeutic escalation. Limitations include the open-label design and heterogeneity in BAT, but the magnitude and persistence of ruxolitinib’s benefit are robust. Future research should focus on optimizing sequencing, duration of therapy, and combination strategies, as well as long-term monitoring for late-onset adverse events.
Conclusion
Ruxolitinib sets a new benchmark for second-line therapy in corticosteroid-refractory or dependent cGVHD, offering durable disease control, steroid-sparing potential, and a favorable long-term safety profile. These findings support its integration into clinical practice and highlight the need for ongoing research into tailored, patient-centric management of chronic GVHD.
References
1. Zeiser R, Russo D, Ram R, Hashmi SK, Chakraverty R, Middeke JM, Musso M, Giebel S, Uzay A, Langmuir P, Hamad N, Burock K, Gowda M, Stefanelli T, Lee SJ, Teshima T, Locatelli F. Ruxolitinib in Patients With Corticosteroid-Refractory or Corticosteroid-Dependent Chronic Graft-Versus-Host Disease: 3-Year Final Analysis of the Phase III REACH3 Study. J Clin Oncol. 2025 Aug 10;43(23):2566-2571. doi: 10.1200/JCO-24-02477 IF: 41.9 Q1 . Epub 2025 Jun 25. PMID: 40561385 IF: 41.9 Q1 ; PMCID: PMC12316163 IF: 41.9 Q1 .2. Jagasia MH, et al. Blood. 2015;125(8):1298-1307.3. European Society for Blood and Marrow Transplantation. Guidelines on cGVHD. 2021.4. U.S. FDA Ruxolitinib Approval Press Release. 2021.