Introduction
Cervical cancer remains a significant global health concern, with early-stage detection offering the potential for curative treatment. Traditional surgical management involves hysterectomy combined with pelvic lymphadenectomy to evaluate lymph node status, which guides post-operative therapy and staging. However, lymphadenectomy is associated with substantial morbidity, including lymphocysts, lymphedema, and nerve injury. The advent of sentinel lymph-node (SLN) biopsy has revolutionized staging in several cancers, notably breast cancer and melanoma, and its application in cervical cancer has been under active investigation.
This article critically examines the recent multicenter, randomized noninferiority trial comparing sentinel-lymph-node biopsy alone versus conventional lymphadenectomy in early-stage cervical cancer. The study aims to determine whether less invasive nodal assessment can maintain oncologic safety while reducing surgical morbidity.
Clinical Background and Unmet Needs
Cervical cancer predominantly affects women worldwide and often presents at an early stage where surgical intervention can be curative. The mainstay of surgical staging has historically involved pelvic lymphadenectomy for accurate nodal status, which influences adjuvant therapy decisions. Nonetheless, lymphadenectomy carries risks that can significantly impair quality of life.
The potential to safely omit lymphadenectomy when sentinel nodes are negative could substantially alter surgical paradigms, reducing complication rates and healthcare costs. Yet, evidence was necessary to establish that such approach does not compromise disease control, prompting the need for rigorous randomized trials.
Study Design and Methodology
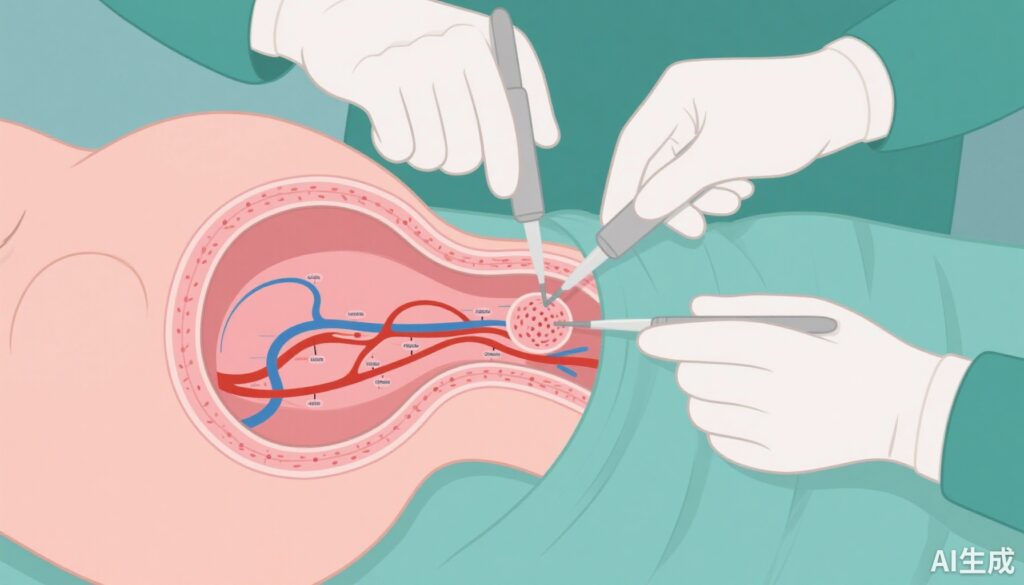
The trial included women with stage IA1 (with lymphovascular invasion), IA2, IB1, or IIA1 cervical cancer, according to the 2009 FIGO classification. Patients underwent sentinel lymph-node biopsy at the time of hysterectomy, with intraoperative frozen section analysis. Those with negative sentinel nodes were randomized intraoperatively in a 1:1 ratio to either omit pelvic lymphadenectomy (biopsy-only group) or proceed with lymphadenectomy.
All participants received hysterectomy, followed by standardized adjuvant therapy as indicated. The primary endpoint was 3-year disease-free survival (DFS), with a predefined noninferiority margin of 5 percentage points. Secondary endpoints included overall survival, nodal recurrences, and surgical complications.
The study enrolled 838 patients with a median follow-up of approximately 63 months, ensuring robust data for long-term survival analysis.
Major Findings and Results
The trial demonstrated that sentinel lymph-node biopsy alone was noninferior to lymphadenectomy in terms of disease-free survival. The 3-year DFS was 94.6% in the lymphadenectomy group and 96.9% in the biopsy-only group.
The difference, -2.3 percentage points, was within the noninferiority margin, with a 95% confidence interval ranging from -5.0 to 0.5 and a P-value < 0.001 for noninferiority. Importantly, cancer-specific survival was high in both groups, with a slightly higher rate in the biopsy-only group (99.2% vs. 97.8%), suggesting the safety of omitting lymphadenectomy.
Recurrence patterns showed no retroperitoneal nodal recurrences in the biopsy-only group, whereas 2.2% of patients in the lymphadenectomy group developed nodal recurrences, indicating that negative sentinel nodes reliably predict the absence of nodal disease.
Regarding morbidity, the biopsy-only group experienced significantly fewer surgical complications, including lymphocysts (8.3% vs. 22.0%), lymphedema (5.2% vs. 19.1%), paresthesia, and pain, underscoring the quality of life benefits associated with less extensive surgery.
Expert Commentary
The findings reinforce the growing role of sentinel lymph-node mapping in managing early-stage cervical cancer. The demonstrated noninferiority in DFS, combined with reduced morbidity, positions sentinel biopsy as a preferable approach in appropriate candidates. However, meticulous technique, experience, and intraoperative assessment are essential to optimize outcomes.
Some limitations include potential false-negative results and the need to ensure proper patient selection. While the study’s results are promising, ongoing research and long-term follow-up are necessary to confirm durability. Current guidelines are gradually incorporating sentinel lymph-node evaluation, but widespread adoption awaits further consensus.
Conclusion
This landmark trial highlights that sentinel-lymph-node biopsy alone is a safe and effective alternative to lymphadenectomy in early cervical cancer, with comparable disease control and markedly fewer complications. Adoption of this approach could redefine surgical standards and improve patient quality of life, marking a significant advancement in oncologic gynecologic surgery.
Funding sources for the study included the Guangzhou Municipal Science and Technology, among others. The trial is registered at ClinicalTrials.gov under the number NCT02642471.
References: Tu H, Huang H, Li Y, et al. Sentinel-Lymph-Node Biopsy Alone or with Lymphadenectomy in Cervical Cancer. NEJM. 2025;393(15):1463-1474. doi: 10.1056/NEJMoa2506267.