Highlights
- Reduced elective neck irradiation (ENI) dose (43 Gy vs. 50 Gy) is non-inferior to standard dosing for head and neck cancer (HNC) in terms of nodal control.
- Lower ENI dose leads to less severe acute swallowing toxicity and better xerostomia-related quality of life.
- Two-year recurrence rates in electively irradiated nodes remain below clinically significant thresholds.
Study Background and Disease Burden
Head and neck squamous cell carcinoma (HNSCC) remains a challenging malignancy, with significant morbidity due to tumor location and the toxicity of curative treatment. Definitive radiotherapy (RT), often with elective neck irradiation (ENI), is a mainstay for organ preservation in HNC. However, ENI—irradiating clinically uninvolved lymphatic regions—substantially contributes to long-term toxicities such as dysphagia (difficulty swallowing) and xerostomia (dry mouth), impacting survivors’ quality of life. Historically, ENI doses have been empirically chosen to maximize regional control, but modern imaging and radiotherapy techniques raise questions as to whether lower doses could safely reduce side effects without compromising disease outcomes.
Study Design
The UPGRADE-RT trial was a prospective, multicenter, randomized controlled study conducted across five Dutch institutions between 2016 and 2022 (ClinicalTrials.gov NCT02442375). Eligible patients had newly diagnosed HNC (clinical stage cT2-4N0-2M0), excluding nasopharyngeal and salivary gland cancers, and were not receiving concurrent chemotherapy.
All patients received definitive accelerated RT to the primary tumor (68 Gy in 34 fractions over 5.5 weeks). ENI dose was randomized in a 2:1 ratio: the dose reduction group received 43 Gy, while the control group received the standard 50 Gy. The primary endpoint was normalcy of diet score at one year, a validated measure of swallowing function and dietary intake. The key secondary endpoint was recurrence rate in electively irradiated lymph nodes at two years. Safety was assessed using toxicity grading and patient-reported quality of life metrics.
Key Findings
A total of 300 patients were randomized, with 295 evaluable for analysis (dose reduction: 196; control: 99). Baseline characteristics were well balanced between groups.
Primary Outcome – Normalcy of Diet
At one year, the mean normalcy of diet score was:
– Dose reduction group: 91.6 (95% CI, 88.5 to 94.7)
– Control group: 92.6 (95% CI, 88.2 to 97.1)
The mean difference was -1.1 (95% CI, -6.5 to 4.4), indicating no clinically meaningful difference in swallowing-related function between the reduced and standard ENI dose arms.
Secondary Outcome – Regional Recurrence
The two-year recurrence rate in electively irradiated nodes was:
– 4.9% (upper-bound one-sided 95% CI, 7.5%) in the dose reduction group
– 4.3% (upper-bound one-sided 95% CI, 7.7%) in the control group
Both rates are well below the predefined safety threshold of 9%, confirming that lower ENI dosing does not substantially increase the risk of regional failure.
Main Outcome Results
| Primary Outcome | Control Group (n = 73) | Dose Reduction Group (n = 146) | Mean Difference (95% CI) | P |
|---|---|---|---|---|
| Normalcy of diet score of PSS-HN, mean score at 1 year (95% CI) | 92.6 (88.2 to 97.1) | 91.6 (88.5 to 94.7) | –1.07 (–6.48 to 4.35) | .698 |
| Secondary outcome | n = 99 | n = 196 | ||
| Recurrence in electively irradiated nodes, 2-year rate (upper-bound one-sided 95% CI) | 4.3 (7.7)a | 4.9 (7.5)a | .005a | |
| Exploratory outcomes | ||||
| Control of disease and survival, 2-year rate (95% CI) | n = 99 | n = 196 | HR (95% CI) | |
| Local control (primary tumor) | 84.5 (77.4 to 92.3) | 81.4 (76.0 to 87.2) | 1.28 (0.69 to 2.40) | .433 |
| Regional control (overall)b | 91.5 (86.0 to 97.3) | 90.4 (86.3 to 94.8) | 1.10 (0.48 to 2.50) | .819 |
| Regional control (intermediate-risk nodes)c | NA | 94.5 (87.1 to 100) | NA | NA |
| Regional control (elective nodes) | 95.7 (91.6 to 99.9) | 95.1 (92.0 to 98.3) | 1.11 (0.34 to 3.60) | .865 |
| Distant control | 91.4 (85.9 to 97.3) | 91.0 (87.0 to 95.2) | 1.06 (0.46 to 2.40) | .899 |
| Recurrence-free survival | 77.5 (69.5 to 86.5) | 73.4 (67.4 to 80.0) | 1.23 (0.74 to 2.00) | .425 |
| Overall survival | 78.7 (71.0 to 87.2) | 83.3 (78.1 to 88.7) | 0.83 (0.49 to 1.40) | .483 |
| Acute toxicity (grade ≥ 3), % | n = 99 | n = 196 | RR (95% CI) | |
| Dermatitis | 7.1 | 6.6 | 0.94 (0.39 to 2.28) | .888 |
| Mucositis | 65.7 | 62.2 | 0.95 (0.79 to 1.13) | .560 |
| Dysphagia (requiring tube feeding) | 19.2 | 10.7 | 0.56 (0.32 to 0.98) | .046 |
| Any of above | 68.7 | 67.3 | 0.98 (0.83 to 1.16) | .815 |
| Late toxicity (grade ≥ 3), 2-year rate (95% CI) | n = 99 | n = 196 | HR (95% CI) | |
| Mucosal ulceration | 4.6 (0.3 to 8.9) | 4.8 (1.7 to 7.9) | 1.06 (0.33 to 3.44) | .923 |
| Laryngeal chondronecrosis | 2.3 (0.0 to 5.4) | 1.1 (0.0 to 2.7) | 0.48 (0.10 to 2.39) | .371 |
| Mandibular osteonecrosis | 1.1 (0.0 to 3.3) | 1.0 (0.0 to 2.4) | 0.98 (0.09 to 10.8) | .985 |
| Any of above | 6.6 (1.5 to 11.7) | 6.3 (2.8 to 9.8) | 0.97 (0.37 to 2.59) | .957 |
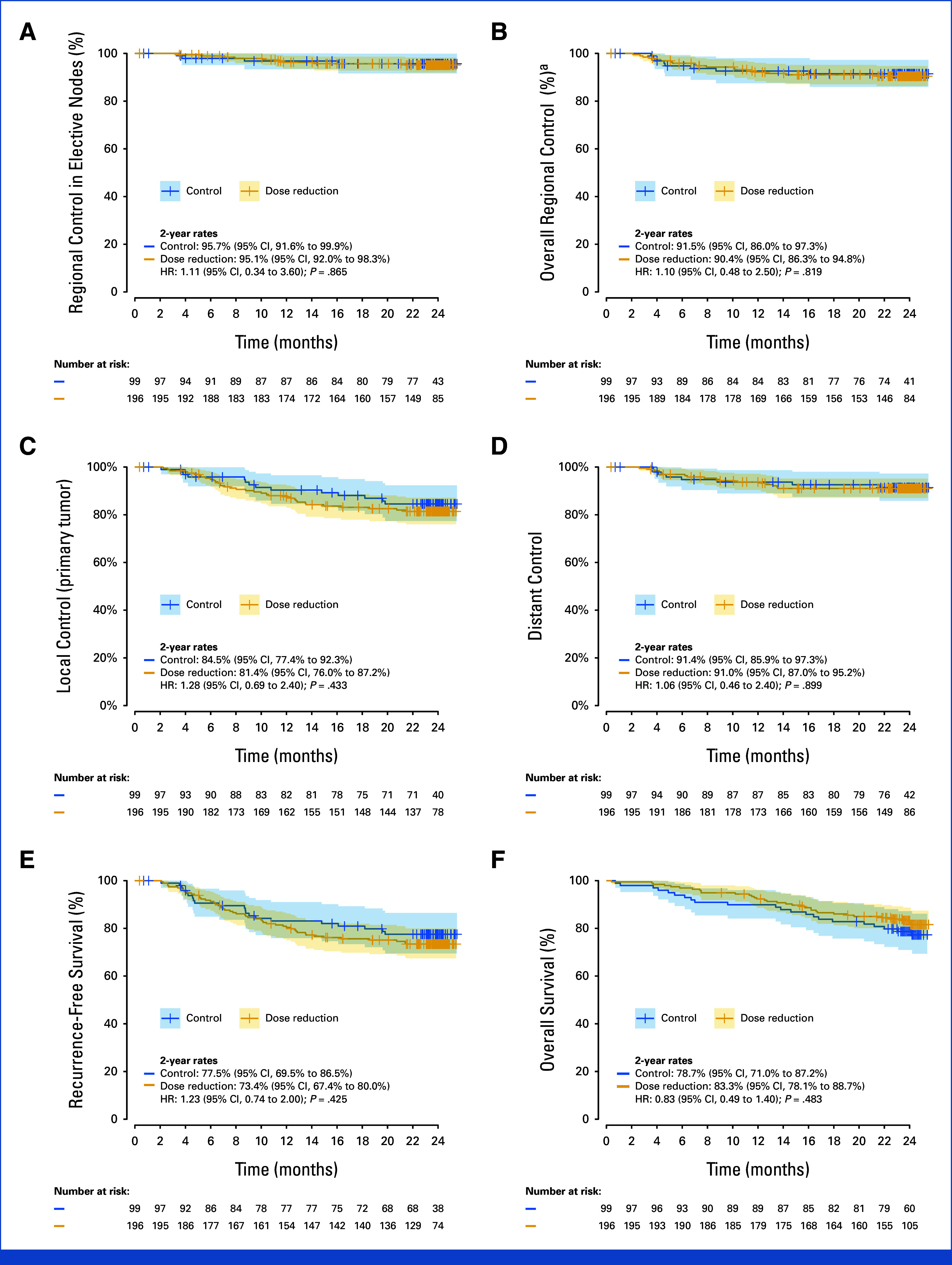
Fig Control of disease and survival
Safety and Quality of Life
Exploratory analyses revealed important differences favoring the reduced dose group:
– Significantly less acute grade ≥3 dysphagia (severe swallowing difficulty)
– Improved xerostomia-related quality of life scores
These findings underscore the clinical importance of lowering elective neck dose, as treatment-related morbidity is a major determinant of long-term patient well-being.
Statistical and Clinical Significance
The trial was powered to detect differences in quality of life and regional control, and the confidence intervals for both primary and secondary endpoints exclude clinically meaningful inferiority of the reduced dose strategy. The absence of increased nodal recurrence, coupled with improved patient-reported outcomes, supports the safety and patient-centered benefit of this approach.
Expert Commentary
The UPGRADE-RT trial is the second randomized controlled study, following the Belgian ESTRO-HNC trial, to rigorously evaluate elective dose de-escalation in HNC. The consistency of findings between trials provides robust evidence that modern radiotherapy planning and target delineation allow safe reduction of ENI dose. As Dr. J.H.A.M. Kaanders and colleagues note, reducing the volume and dose of irradiated normal tissue can mitigate some of the most disabling late toxicities, without sacrificing disease control. However, patient selection remains critical—these results apply to patients similar to those enrolled (excluding concurrent chemoradiation and advanced nodal disease).
Limitations include the exclusion of patients receiving concurrent chemotherapy and those with more advanced nodal involvement (N3 disease), which may limit generalizability. Additionally, longer-term follow-up is required to assess late toxicity and very late recurrences.
Conclusion
The UPGRADE-RT trial provides compelling evidence that reduced elective neck irradiation dose (43 Gy) is safe and confers important quality of life benefits compared to standard dosing in definitive radiotherapy for HNC. These results support a re-evaluation of ENI dosing in clinical practice, with the potential to improve survivorship outcomes for a substantial proportion of patients. Further research should explore the application of dose de-escalation in broader patient cohorts and in combination with systemic therapies.
References
1. van den Bosch S, Doornaert PAH, Hoebers FJP, Kreike B, Vergeer MR, Zwijnenburg EM, Cox MC, Hannink G, Dijkema T, Kaanders JHAM; Dutch Head and Neck Society. Clinical Benefit and Safety of Reduced Elective Dose in Definitive Radiotherapy for Head and Neck Squamous Cell Carcinoma: The UPGRADE-RT Multicenter Randomized Controlled Trial. J Clin Oncol. 2025 Aug 10;43(23):2583-2594. doi: 10.1200/JCO-24-02194 IF: 41.9 Q1 . Epub 2025 Apr 15. PMID: 40233286 IF: 41.9 Q1 ; PMCID: PMC12316137 IF: 41.9 Q1 . PDF (841.1 KB)
2. Gregoire V, et al. Elective nodal irradiation in head and neck cancer: Where do we stand? Radiother Oncol. 2021;156:209-216.3. Nutting CM, et al. Reducing radiotherapy-related toxicity in head and neck cancer. Br J Cancer. 2017;117(3):293-299.