Highlight
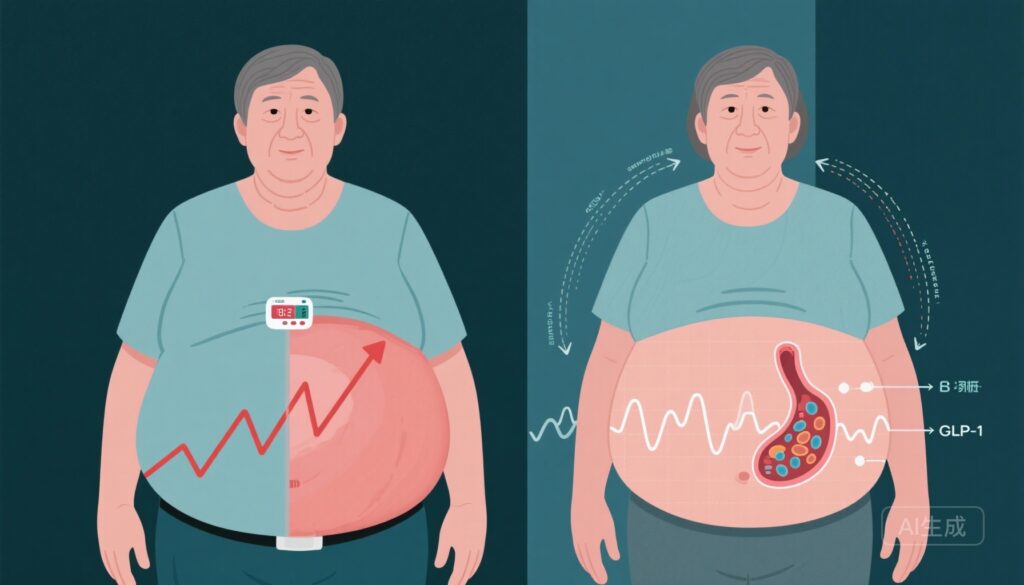
– Prediabetes remission (return to normal glucose regulation) can be achieved without overall weight loss and substantially reduces progression to type 2 diabetes (T2D).
– Mechanisms include improved insulin sensitivity, enhanced β‑cell function and increased β‑cell responsiveness to GLP‑1, with differential adipose redistribution (subcutaneous instead of visceral).
– These findings from a post hoc analysis of the multicenter Prediabetes Lifestyle Intervention Study (PLIS) were reproduced in the US Diabetes Prevention Program (DPP), prompting reconsideration of guideline targets that focus on weight loss alone.
Background: unmet need in diabetes prevention
Prediabetes affects a large and growing fraction of adults worldwide and identifies people at substantially increased short-term risk for type 2 diabetes. Clinical practice guidelines and major prevention programmes (for example, the Diabetes Prevention Program, DPP) have historically prioritized structured lifestyle interventions aiming for defined weight-loss targets (commonly ~5–7% body weight) plus increased physical activity. Those approaches reduce T2D incidence, but many at-risk individuals find sustained weight loss difficult to achieve, and residual diabetes risk remains.
The concept of remission — returning glycaemia to normal ranges defined by the American Diabetes Association (ADA) — has risen to prominence for established T2D in the context of weight-loss interventions (surgical or intensive lifestyle). Less attention has been paid to using remission as an explicit target in people with prediabetes. The post hoc analysis of the Prediabetes Lifestyle Intervention Study (PLIS) reported by Sandforth et al. (Nat Med. 2025) suggests that aiming for glycemic normalization may prevent progression to T2D even when weight loss is not achieved, challenging the primacy of body-weight goals alone.
Study design and comparators
PLIS was a large, multicenter, randomized controlled trial of lifestyle interventions in individuals with prediabetes. The present article reports a post hoc analysis of PLIS in which participants were categorized as responders (those who achieved prediabetes remission during follow-up using ADA criteria) or nonresponders. Key metabolic phenotyping included measures of insulin sensitivity, β‑cell function, assessments of adipose tissue compartments by imaging, and incretin responsiveness (β‑cell–GLP‑1 sensitivity).
The authors also replicated key observations in the US Diabetes Prevention Program (DPP) cohort to test generalizability. The DPP is a long-standing randomized trial comparing intensive lifestyle intervention, metformin, and placebo for T2D prevention, with a well-characterized dataset allowing secondary analyses.
Key findings
1. Prediabetes remission occurred without overall weight loss
The PLIS post hoc analysis demonstrated that a meaningful subset of participants met ADA criteria for prediabetes remission despite no net weight loss and, in some cases, modest weight gain. Responders and nonresponders showed similar overall weight change distributions, indicating that normalization of glucose does not require the typical 5–7% weight-loss threshold to be achieved in all individuals.
2. Remission associated with reduced progression to type 2 diabetes
Participants who achieved remission had substantially lower incidence of subsequent T2D compared with nonresponders. The protective effect of remission was independent of weight change, supporting the clinical relevance of glycemic normalization as an outcome in itself. These protective associations were reproduced in analyses of the DPP cohort, increasing confidence in external validity.
3. Metabolic mechanisms: insulin sensitivity, β‑cell function and GLP‑1 responsiveness
Detailed metabolic phenotyping revealed that remission was accompanied by improvements in whole-body insulin sensitivity and enhancements in β‑cell secretory capacity. Importantly, responders exhibited increases in β‑cell–GLP‑1 sensitivity, suggesting improved incretin action as part of the mechanistic signature associated with remission. This constellation of changes provides a plausible biological pathway by which glycemia can normalize even without net loss of body weight.
4. Adipose tissue redistribution: subcutaneous vs visceral
Although overall weight change did not differ significantly between responders and nonresponders, body-fat compartments diverged. Nonresponders tended to increase visceral adipose tissue (VAT) mass, a depot strongly linked to insulin resistance and cardiometabolic risk. In contrast, responders preferentially increased subcutaneous adipose tissue (SAT) depots (or had relatively greater SAT vs VAT), a pattern that is metabolically less harmful. The authors propose that favorable adipose redistribution may underlie improved insulin sensitivity and β‑cell function in the absence of net weight loss.
5. Replication in DPP
Key observations—remission without weight loss, its protective association with lower T2D incidence, and metabolic correlates—were reproduced in secondary analyses of the DPP cohort, enhancing the generalizability of the findings across different trial settings and populations.
Expert commentary and interpretation
These analyses introduce a paradigm shift in diabetes prevention: glycemic remission (normalization) should be considered an explicit and achievable target independent of weight loss in at least a subset of people with prediabetes. Mechanistically, the findings are coherent with established physiology: reduced VAT favors better hepatic and systemic insulin sensitivity; improved incretin responsiveness enhances glucose-stimulated insulin secretion; combined, these effects can normalize glycaemia even if total adiposity remains unchanged.
From a clinical standpoint, the results indicate that focusing solely on percent weight loss may miss alternative, clinically meaningful paths to risk reduction. They also suggest that interventions emphasizing diet quality, timing of meals, physical activity modalities that improve insulin action, and strategies that favor subcutaneous rather than visceral fat storage could be beneficial—even when they do not produce large sustained weight losses.
The observed increase in β‑cell–GLP‑1 sensitivity in responders raises intriguing translational questions. GLP‑1 receptor agonists are potent glucose-lowering and weight-loss agents; these data suggest that enhancing incretin action might contribute to remission pathways beyond weight loss. However, the PLIS analyses do not evaluate pharmacologic GLP‑1 therapy and do not imply equivalence between drug-induced weight loss and the metabolic improvements seen here.
Limitations and cautions
Key limitations to bear in mind include the post hoc nature of the PLIS analysis: responder classification and mechanistic inference were not primary randomized comparisons specified a priori. Residual confounding and selection biases can influence secondary analyses. Although replication in the DPP reduces the chance that findings are idiosyncratic, prospective trials designed to test glycemic-remission–focused interventions are needed to confirm causality.
Another limitation is that the detailed metabolic phenotyping described in PLIS may not be available in routine clinical care. Operationalizing remission as a guideline target will therefore require pragmatic approaches to measurement (for example, HbA1c and fasting glucose, or targeted OGTTs) and clear recommendations on monitoring frequency and intervention steps if remission is not achieved.
Finally, safety and long-term outcomes beyond diabetes incidence (for example cardiovascular events, microvascular complications, and quality of life) were not the primary focus of these analyses; those endpoints require longer-term study.
Implications for practice and guidelines
The data argue for revising prevention frameworks to include glycemic normalization as an explicit objective alongside, or in some patients instead of, percent weight-loss goals. Practical implications could include:
- Routine measurement and documentation of glycemic status (fasting glucose, HbA1c, and use of OGTT where indicated) with explicit documentation of remission when ADA criteria are met.
- Personalized counseling that emphasizes not only weight loss but also interventions most likely to improve insulin sensitivity and β‑cell function (diet composition and timing, physical activity types that boost insulin sensitivity, sleep and stress optimization).
- Research and guideline development to define standardized remission-focused care pathways, monitoring intervals, and escalation steps (behavioral, pharmacologic, or combined).
In particular, clinical trials that randomize participants to interventions explicitly aimed at glycemic remission (with glycemia as the primary endpoint) are needed to confirm that remission-focused strategies reduce T2D incidence and improve patient-centered outcomes.
Research priorities
Priority research questions include:
- Prospective trials targeting remission as a prespecified endpoint to compare remission-focused behavioural programmes vs standard weight-loss–focused programmes.
- Mechanistic studies to dissect the contributions of adipose tissue redistribution, muscle insulin sensitivity, hepatic glucose production, and incretin signaling to remission.
- Health services research to determine scalable ways to measure and achieve remission in diverse populations, including the role of digital health, community programs, and pharmacotherapy adjuncts.
Conclusion
The PLIS post hoc analysis, with replication in the DPP, shows that prediabetes remission is achievable in some individuals without net weight loss and that remission markedly reduces progression to type 2 diabetes. Mechanistic signals implicate improved insulin sensitivity, augmented β‑cell function and β‑cell–GLP‑1 responsiveness, and favorable adipose redistribution from visceral toward subcutaneous stores. These findings support a broader prevention paradigm in which glycemic normalization—not only percent body-weight change—serves as an actionable target. Prospective trials and guideline deliberations should evaluate how to integrate glycemic‑remission goals into routine diabetes-prevention practice.
Funding and clinicaltrials.gov
Funding sources, trial registration numbers and detailed acknowledgements are reported in the original publication: Sandforth A et al., Nat Med. 2025. Readers should consult that article for trial-specific funding and registration details.
References
1. Sandforth A, Arreola EV, Hanson RL, et al. Prevention of type 2 diabetes through prediabetes remission without weight loss. Nat Med. 2025 Oct;31(10):3330–3340. doi:10.1038/s41591-025-03944-9.
2. Diabetes Prevention Program Research Group; Knowler WC, Fowler SE, Hamman RF, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002 Feb 7;346(6):393–403.
3. Lean MEJ, Leslie WS, Barnes AC, et al. Primary care-led weight management for remission of type 2 diabetes (DiRECT): an open-label, cluster-randomised trial. Lancet. 2018;391(10120):541–551.
4. American Diabetes Association. Standards of Medical Care in Diabetes—2023. Diabetes Care. 2023;46(Suppl 1):S1–S354.
Acknowledgement
This article synthesizes and critically interprets findings reported by Sandforth et al. (2025) and places them in clinical and research context. The author declares no conflicts of interest related to this summary.