Highlights
- LBM-based oxaliplatin dosing reduced grade ≥2 peripheral neurotoxicity compared to standard dosing.
- No compromise in relapse-free or overall survival when using LBM-adjusted doses.
- Quality of life was improved, and fewer dose reductions were needed in the LBM-based group.
Study Background and Disease Burden
Colorectal cancer is among the leading causes of cancer-related morbidity and mortality worldwide. For patients with stage III colon cancer, adjuvant chemotherapy with oxaliplatin-based regimens (commonly FOLFOX: fluorouracil, leucovorin, and oxaliplatin) is standard of care and has substantially improved survival rates. However, oxaliplatin-induced peripheral neurotoxicity (OIPN), manifesting as sensory deficits, pain, and functional impairment, remains a major dose-limiting and often disabling side effect. Cumulative neurotoxicity can lead to treatment interruptions, reduced chemotherapy intensity, and long-term quality of life impairment. Traditionally, oxaliplatin dosing is based on body surface area (BSA), but this approach does not account for inter-individual variability in body composition—particularly lean body mass (LBM)—which influences drug pharmacokinetics and toxicity risk. Prior analyses suggested that doses exceeding 3.09 mg/kg LBM are associated with increased neurotoxicity, raising the question of whether personalized dosing could mitigate this risk.
Study Design
The LEANOX trial (NCT03255434) was a multicenter, phase II, randomized proof-of-concept study aiming to determine whether LBM-based oxaliplatin dosing reduces neurotoxicity in the adjuvant treatment of resected stage III colon cancer. Patients eligible for adjuvant FOLFOX were stratified based on LBM status:
– Arm 1: Patients without LBM reduction received standard BSA-based oxaliplatin dosing (85 mg/m2).
– Arms 2 and 3: Patients with reduced LBM were randomized 1:1 to either BSA-based dosing (Arm 2) or LBM-based dosing (3.09 mg/kg LBM, Arm 3).
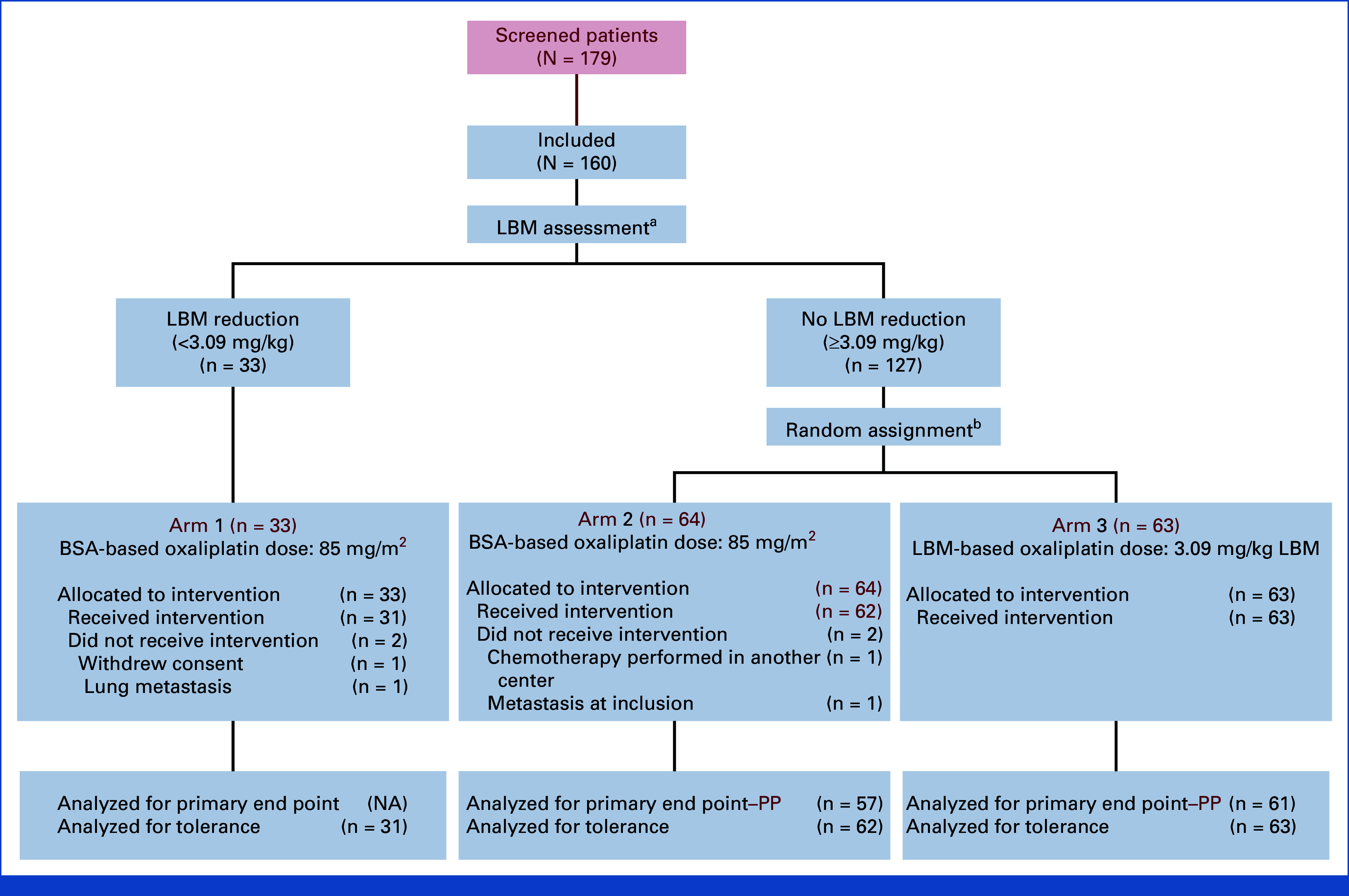
The primary endpoint was the proportion of patients free from grade ≥2 OIPN during the first six chemotherapy cycles. Secondary endpoints included time to onset of grade ≥2 OIPN, cumulative oxaliplatin dose delivered without neurotoxicity, dose reductions, relapse-free survival (RFS), overall survival (OS), and patient-reported quality of life (using the QLQ-CIPN20 instrument).
A total of 160 patients were enrolled (33 in arm 1, 64 in arm 2, 63 in arm 3), with a median age of 63 years and balanced demographic/clinical characteristics across arms. Median follow-up was 38.6 months.
Key Findings
The primary endpoint—absence of grade ≥2 OIPN—was achieved by 67.2% of patients in the LBM-adjusted arm (arm 3) compared to only 42.1% in the BSA-based, reduced-LBM arm (arm 2), a statistically significant difference (P = .01). Importantly, this benefit was not observed in patients without LBM reduction (arm 1), underscoring the value of individualized dosing in a susceptible subgroup.
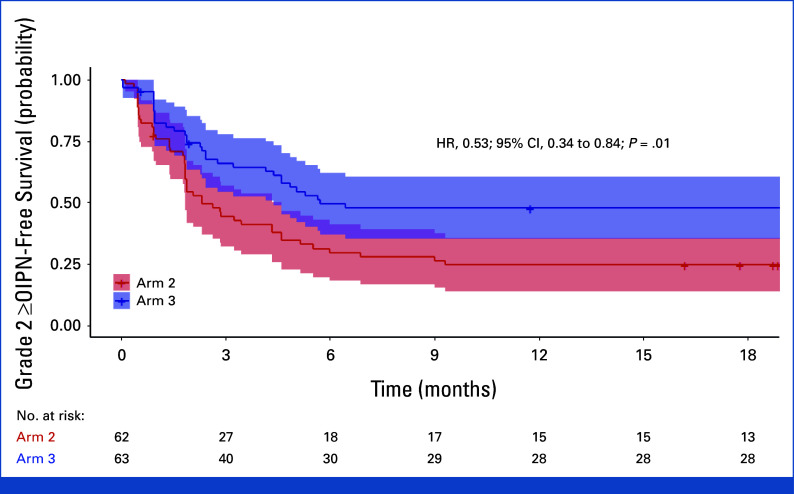
Kaplan-Meir estimates of grade ≥2 OIPN-free survival. Kaplan-Meier estimates of grade ≥2 OIPN-free survival in arm 2 (BSA-based dose) and arm 3 (LBM-based dose). The shaded regions represent the 95% CIs. HRs were calculated using the Cox proportional hazards model, with 95% CI based on log transformation and exponentiation for CI estimation, and P values determined with the log-rank test. BSA, body surface area; HR, hazard ratios; LBM, lean body mass; OIPN, oxaliplatin-induced peripheral neuropathy.
Additional efficacy and safety findings included:
– **Longer OIPN-Free Survival**: Arm 3 demonstrated a significantly longer duration before the onset of grade ≥2 OIPN (hazard ratio [HR], 0.53; 95% CI, 0.34–0.84; P = .01).
– **Delayed Neurotoxicity**: The time to first occurrence of grade ≥2 OIPN was significantly delayed in arm 3 (P = .006).
– **Higher Delivered Dose Intensity**: Patients in the LBM-based group received higher cumulative oxaliplatin doses without significant neurotoxicity (P = .044), indicating that dose intensity could be safely maintained.
– **Fewer Reductions/Interruptions**: The frequency of oxaliplatin dose reductions was markedly lower in arm 3 (P < .001).
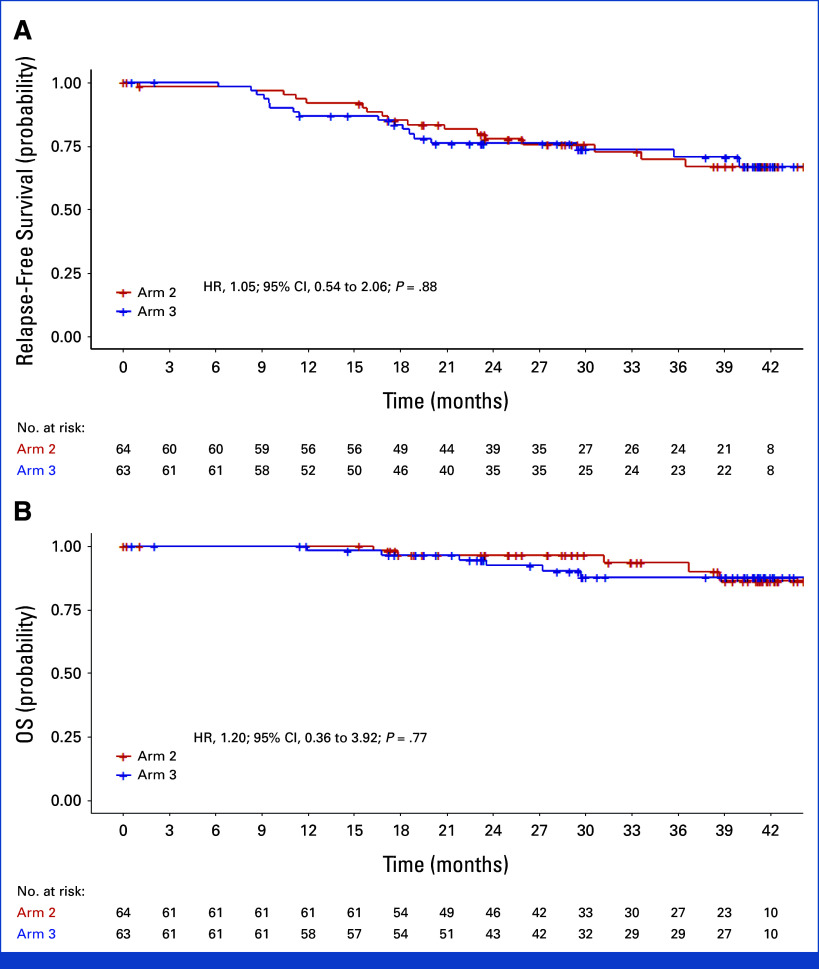
– **Relapse-Free Survival & Overall Survival**: There were no statistically significant differences in RFS (HR, 1.05 [95% CI, 0.54–2.06]) or OS (HR, 1.20 [95% CI, 0.36–3.92]) between arms 2 and 3. These data support the oncologic safety of LBM-based dose reduction.
– **Quality of Life**: Patient-reported neuropathy symptoms (QLQ-CIPN20 scores) were significantly better in the LBM-based group, indicating meaningful improvements in daily functioning and well-being.
Kaplan-Meir estimates of relapse-free survival and OS. Kaplan-Meier estimates of (A) relapse-free survival and (B) OS in arm 2 (BSA-based oxaliplatin dose) and arm 3 (LBM-based oxaliplatin dose).
Expert Commentary
The LEANOX trial provides compelling evidence that LBM-based dosing of oxaliplatin is a rational, safe, and effective strategy to reduce the burden of chemotherapy-induced neurotoxicity in a well-defined subgroup of stage III colon cancer patients. This approach addresses a critical unmet need: preserving quality of life and functional status without undermining the curative potential of adjuvant therapy. The trial’s strengths include a robust randomized design, clear patient stratification, and clinically meaningful endpoints. Notably, survival outcomes were not compromised, allaying concerns that dose reduction might diminish efficacy.
However, some limitations warrant consideration. The trial was phase II and focused on proof-of-concept rather than definitive practice change; larger phase III studies will be required to confirm these findings and support guideline integration. The need for accurate LBM assessment (often via CT or DEXA scan) may pose logistical challenges in routine oncology practice. Furthermore, the generalizability to diverse populations and other chemotherapy regimens remains to be established.
Mechanistically, these findings are biologically plausible: LBM is a key determinant of drug distribution and clearance, and leaner patients may be at disproportionate risk for toxicity when exposed to BSA-based dosing. Personalizing cytotoxic chemotherapy according to body composition represents a promising step toward precision oncology.
Conclusion
The phase II LEANOX trial demonstrates that LBM-based oxaliplatin dosing can significantly reduce neurotoxicity and improve patient quality of life, with no adverse impact on relapse-free or overall survival in the adjuvant treatment of stage III colon cancer. These results support further research and consideration of LBM-based dose adjustment as a strategy to optimize adjuvant chemotherapy tolerability and effectiveness. Broader adoption may depend on developing streamlined, accessible methods for LBM assessment in clinical practice.
References
1. Assenat E, Ben Abdelghani M, Gourgou S, Perrier H, Akouz FK, Desgrippes R, Galais MP, Janiszewski C, Mazard T, Rinaldi Y, Lepage C, Tetreau R, Senesse P. Impact of Lean Body Mass-Based Oxaliplatin Dose Calculation on Neurotoxicity in Adjuvant Treatment of Stage III Colon Cancer: Results of the Phase II Randomized LEANOX Trial. J Clin Oncol. 2025 Aug 10;43(23):2616-2627. doi: 10.1200/JCO-24-02754 IF: 41.9 Q1 2. André T, et al. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med. 2004;350:2343-2351.
3. Grothey A, Sobrero AF, et al. Duration of adjuvant chemotherapy for stage III colon cancer. N Engl J Med. 2018;378:1177-1188.