Highlights
- Adequate daptomycin dosing led to a 91% clinical success rate in Japanese pediatric patients.
- Underdosing was strongly associated with higher mortality, particularly in younger children.
- Adverse events related to daptomycin occurred regardless of renal function, necessitating vigilant monitoring.
- Renal function did not predict daptomycin-related adverse events in this cohort.
Study Background and Disease Burden
Daptomycin, a cyclic lipopeptide antibiotic, is a critical agent for managing severe Gram-positive infections, including methicillin-resistant Staphylococcus aureus (MRSA), in both adult and pediatric populations. However, pediatric pharmacokinetics differ markedly from adults, and the safety and effectiveness of daptomycin in children remain less well characterized, particularly in Asian populations. Considering that renal function and appropriate dosing are central to optimizing antimicrobial therapy and minimizing adverse effects, there is a significant unmet need for robust data guiding daptomycin use in pediatric patients, especially in settings where multidrug-resistant infections are prevalent.
Study Design
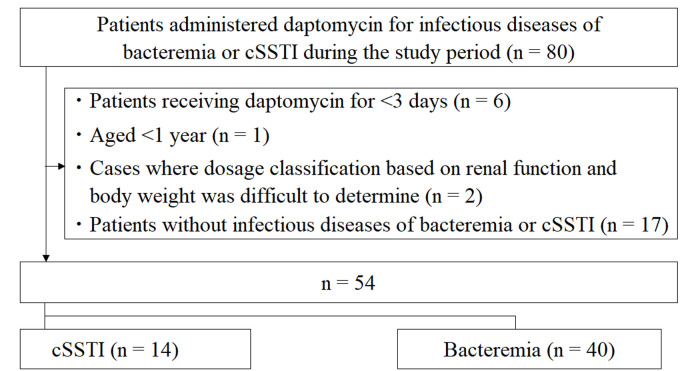
This retrospective, multicenter observational study evaluated pediatric patients treated with daptomycin across multiple Japanese institutions between September 2011 and December 2022. Inclusion criteria were pediatric age and receipt of at least one dose of daptomycin within the study period. Data were extracted from medical records, and clinical effectiveness was categorized as cure, improvement, failure, or non-evaluable. The clinical success rate was defined as the combined proportion of cured and improved patients.
Daptomycin dosing was classified as underdose (>1 mg/kg less than the approved pediatric dose), adequate dose (approved dose ±1 mg/kg), or overdose (>1 mg/kg more than the approved dose). For obese patients (BMI ≥ 30 kg/m²), adjusted body weight was used for dosing calculations. The study also assessed adverse events attributed to daptomycin, including muscle toxicity (creatine phosphokinase elevation, rhabdomyolysis), pulmonary toxicity (eosinophilic pneumonia), hepatotoxicity (liver failure), and drug fever. Renal function was analyzed as a potential risk factor for adverse events and treatment outcomes.
Flowchart of the inclusion and exclusion of patients in this study.
Key Findings
A total of 54 pediatric patients were included in the analysis. The following summarizes the main results:
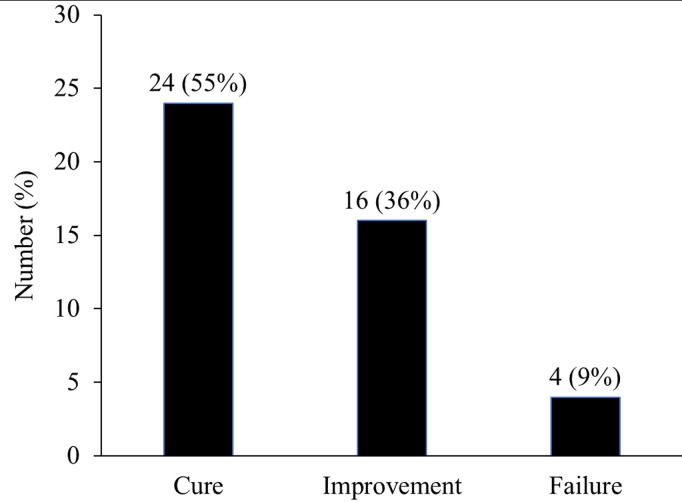
- Clinical Effectiveness: The overall clinical success rate was 91%, indicating high therapeutic efficacy for daptomycin in this real-world pediatric cohort.
- Mortality and Dosing: Four patients (7%) died during the study. Mortality was significantly higher among those with microbiological failure (P = 0.004) and in the underdose group (P < 0.001). Notably, younger patients were more likely to be underdosed (P = 0.020), suggesting a need for age- and weight-adjusted dosing vigilance.
-
Clinical effectiveness.
- Adverse Events: Seven patients (13%) experienced daptomycin-related adverse events. These included creatine phosphokinase elevation (indicative of muscle injury), eosinophilic pneumonia, rhabdomyolysis, liver failure, and drug fever. Importantly, adverse events were observed in patients receiving both adequate and excessive doses and occurred irrespective of underlying renal function.
- Renal Function: Contrary to concerns about nephrotoxicity, the incidence of adverse events did not correlate with renal impairment in this cohort. However, continuous safety monitoring remains warranted, given the spectrum of potential daptomycin toxicities.
| Group | Number of Patients | Clinical Success Rate | Adverse Event Rate | Mortality |
|---|---|---|---|---|
| Underdose | – | Lower (significant) | – | Higher |
| Adequate Dose | – | High | Present | Low |
| Overdose | – | – | Present | – |
Expert Commentary
This study addresses a crucial gap in pediatric infectious disease pharmacotherapy, offering robust multicenter data on daptomycin’s efficacy and safety in Japanese children. The findings reinforce the need for strict adherence to approved dosing guidelines—particularly in younger and obese children—to optimize outcomes and minimize mortality. The lack of association between renal function and adverse event risk is somewhat reassuring but does not eliminate the need for ongoing monitoring, given the observed spectrum of toxicities. These results are consistent with recent international guidelines that recommend individualized dosing strategies in pediatric antimicrobials and underscore the importance of therapeutic drug monitoring, especially for agents with narrow therapeutic indices or variable pharmacokinetics in children (see Infectious Diseases Society of America guidelines).
Study limitations include the retrospective design, relatively small sample size, and lack of a formal control group. Nevertheless, the high clinical success rate and clear linkage between underdosing and poor outcomes provide actionable insights for clinicians.
Conclusion
This multicenter retrospective study demonstrates that adequate dosing of daptomycin is critically important for achieving high clinical effectiveness and reducing mortality in Japanese pediatric patients. Adverse events, although not predicted by renal function, underline the necessity for continuous clinical and laboratory monitoring during therapy—even at approved doses. These findings should inform local antimicrobial stewardship programs and highlight the need for further prospective research to refine pediatric dosing strategies for daptomycin and other critical antibiotics.
References
1. Shiraishi C, Kato H, Hirai J, et al. Influence of renal function and daptomycin dose on clinical effectiveness and adverse events in Japanese pediatric patients: A multicenter retrospective observational study. PLoS One. 2025 Jul 17;20(7):e0327993. doi: 10.1371/journal.pone.0327993 IF: 2.6 Q2 . PMID: 40674361 IF: 2.6 Q2 ; PMCID: PMC12270112 IF: 2.6 Q2 .
2. Infectious Diseases Society of America (IDSA) Clinical Practice Guidelines for the Treatment of Methicillin-Resistant Staphylococcus aureus Infections in Adults and Children. Clin Infect Dis. 2011;52(3):e18-e55.