Introduction
Sensorineural hearing loss (SNHL) afflicts over 1.5 billion people worldwide and represents a leading cause of disability. Its multifactorial etiology encompasses genetic mutations, environmental insults such as noise exposure, ototoxic drugs, aging, and viral infections. Increasing evidence implicates the NOD-like receptor pyrin domain-containing 3 (NLRP3) inflammasome as a pivotal driver of inflammation-induced cochlear damage underlying many SNHL forms. The dysregulated activation of this innate immune complex triggers a cascade of proinflammatory cytokine release and pyroptotic cell death, contributing to progressive and often irreversible auditory dysfunction. This article critically reviews the mechanistic roles of the NLRP3 inflammasome in diverse hearing loss etiologies and highlights emerging therapeutic candidates targeting this pathway with translational potential.
NLRP3 Inflammasome Biology and Activation in the Cochlea
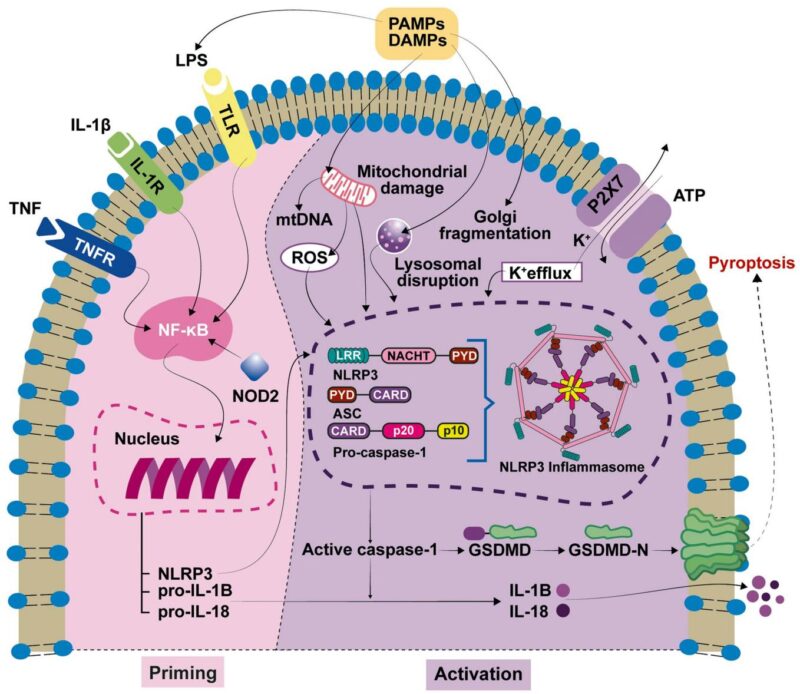
The NLRP3 inflammasome is a cytosolic multiprotein complex comprising the NLRP3 sensor protein, the adaptor ASC, and pro-caspase-1, acting as an intracellular sentinel for pathogenic and sterile danger signals. Activation involves a two-step process: a priming signal, often mediated by pattern recognition receptors (such as TLRs) recognizing pathogen- or damage-associated molecular patterns (PAMPs and DAMPs), which induces transcriptional upregulation of inflammasome components and proinflammatory cytokines pro-IL-1β and pro-IL-18; and an activation step triggered by various stimuli including ion flux, reactive oxygen species, and mitochondrial damage leading to NLRP3 oligomerization, ASC recruitment, caspase-1 activation, and cleavage of pro-IL-1β/IL-18 into bioactive cytokines. Caspase-1 also cleaves gasdermin D (GSDMD), whose N-terminal fragment forms pores in the plasma membrane resulting in pyroptotic cell death and cytokine release.
In the cochlea, NLRP3 inflammasome components are expressed largely in resident macrophages, marginal cells of the stria vascularis, supporting cells, and spiral ganglion neurons, placing this innate immune platform at the nexus of cochlear homeostasis and inflammatory pathology. Activation of NLRP3 induces secretion of IL-1β and IL-18 which propagate inflammation and may compromise the delicate cochlear microenvironment essential for hair cell and neuronal function.
Role of NLRP3 Inflammasome in Hearing Loss Etiologies
Genetic Forms of Hearing Loss and Autoinflammatory Syndromes
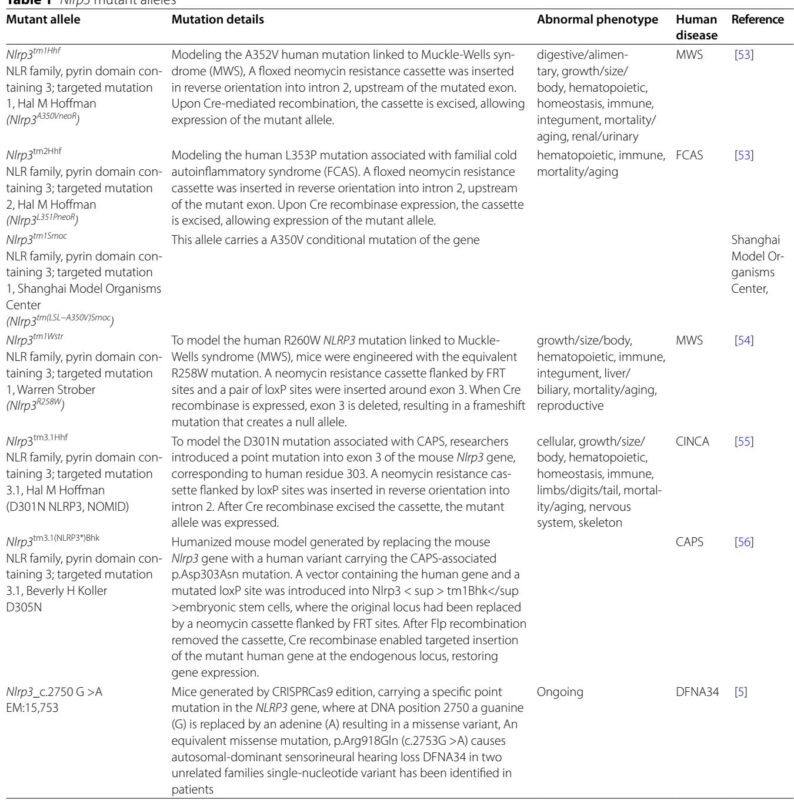
Mutations in the NLRP3 gene cause cryopyrin-associated periodic syndromes (CAPS), encompassing familial cold autoinflammatory syndrome (FCAS), Muckle-Wells syndrome (MWS), and chronic infantile neurological cutaneous and articular syndrome (CINCA), which often feature sensorineural hearing loss. Gain-of-function mutations drive excessive IL-1β production, systemic inflammation, and cochlear autoinflammation. The prevalence of CAPS is low but likely underestimated due to diagnostic delays. Genetic analyses, including targeted and whole-exome sequencing, reveal that certain NLRP3 variants predominantly manifest with hearing loss without overt systemic symptoms (classified as DFNA34), underscoring the spectrum of inflammatory hearing phenotypes.
Animal models expressing patient-derived NLRP3 mutations have validated the pathogenic role of inflammasome activation in cochlear inflammation and hearing deficits, though severe systemic effects limit early auditory phenotyping in some models. Conditional mouse models with targeted cochlear expression of mutant NLRP3 exhibit profound hearing loss associated with inflammation and cochlear architectural disruption, recapitulating human disease features.
Acquired and Environmental Hearing Loss
NLRP3 inflammasome activation has been implicated experimentally in noise-induced hearing loss (NIHL), age-related hearing loss (ARHL), and ototoxic drug-induced auditory damage. Noise exposure induces cochlear release of DAMPs, triggering inflammasome assembly and elevated IL-1β/IL-18, with pharmacological inhibition conferring protection in preclinical models. ARHL correlates with elevated cochlear inflammasome activation, suggesting chronic low-grade inflammation contributes to age-associated auditory decline.
Cisplatin and aminoglycoside antibiotics induce ototoxicity partly via NLRP3-dependent activation, promoting pyroptosis in cochlear hair and marginal cells. Notably, agents such as oridonin and MCC950 ameliorate ototoxic injury by blocking inflammasome assembly and downstream signaling. Viral infections (e.g., cytomegalovirus) can also trigger inflammasome-mediated cochlear neuronal death, contributing to congenital SNHL.
Other Inner Ear Pathologies
Emerging data link NLRP3 inflammasome activation to Meniere’s disease and vestibular schwannoma-associated hearing loss, highlighting its broader role in inner ear inflammatory processes influencing auditory function.
Therapeutic Targeting of NLRP3 Inflammasome in Hearing Loss
Current FDA-approved therapies for CAPS include IL-1 pathway blockers such as anakinra (IL-1R antagonist) and canakinumab (anti-IL-1β monoclonal antibody), which can stabilize or improve hearing outcomes, particularly when initiated early. Rilonacept and experimental monoclonal antibodies like gevokizumab are under clinical evaluation.
Preclinical and early clinical progress of direct NLRP3 inhibitors, including MCC950 and dapansutrilo (OLT1177), offer promising avenues for broader SNHL treatment beyond CAPS. Natural compounds such as piceatannol and oridonin exhibit immunomodulatory and otoprotective effects by suppressing inflammasome activation and pyroptosis in models of ARHL, NIHL, and drug-induced ototoxicity.
Drug repurposing efforts, exemplified by tranylcypromine’s inhibitory effects on inflammasome signaling, expand potential therapeutic options. Cochlear implantation remains an important intervention for advanced cases unresponsive to pharmacotherapy.
Conclusions and Future Directions
The NLRP3 inflammasome emerges as a central pathogenic mediator of SNHL of diverse origins through its regulation of cochlear inflammation and cell death pathways. Genetic and experimental data converge to support its therapeutic targeting to prevent or halt progressive hearing loss. Novel selective NLRP3 inhibitors currently in clinical development herald a new class of targeted therapeutics with potential to transform management. Future research priorities include validation of these agents in human clinical trials, identification of reliable biomarkers for early detection, and exploration of combinatorial strategies integrating anti-inflammatory, antioxidant, and regenerative therapies to optimize hearing preservation.
Advances in molecular imaging and animal modeling will accelerate mechanistic insights, facilitating translation of inflammasome-targeted therapies into clinical practice and offering hope to millions affected by debilitating hearing loss.
Reference:
Murillo-Cuesta S, Seoane E, Cervantes B, Zubeldia JM, Varela-Nieto I. NLRP3 inflammasome and hearing loss: from mechanisms to therapies. J Neuroinflammation. 2025 Oct 4;22(1):225. doi: 10.1186/s12974-025-03561-w. PMID: 41046290; PMCID: PMC12497346.



