Introduction
Autism spectrum disorder (ASD) is a complex neurodevelopmental condition characterized by impairments in social communication, interaction, and the presence of restrictive and repetitive behaviors. While ASD’s etiology remains incompletely understood, disturbances in gut microbiota—referred to as dysbiosis—have emerged as intriguing contributors to both gastrointestinal (GI) and neurobehavioral symptoms in affected individuals. Children with ASD frequently experience GI comorbidities such as constipation, diarrhea, and abdominal discomfort, which can exacerbate behavioral challenges and impact overall quality of life. Fecal microbiota transplantation (FMT), the transfer of stool-derived microorganisms from healthy donors to recipients, has gained attention as a potential intervention targeting gut dysbiosis to ameliorate ASD symptoms. This article systematically reviews the current clinical evidence regarding FMT’s efficacy and safety in children and adolescents with ASD, synthesizing findings from randomized controlled trials (RCTs) and observational studies to inform clinical practice and future research.
Study Design
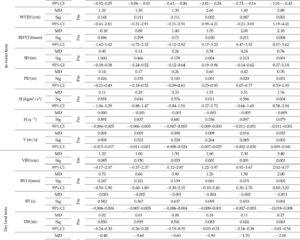
This review includes two double-blind, placebo-controlled RCTs and seven before-and-after studies evaluating FMT in pediatric ASD populations. Participants ranged in age from 2 to 17 years, predominantly male, and were diagnosed based on standard diagnostic criteria (DSM-5, ADOS-2, or ADI-R). Interventions varied widely in FMT preparation (lyophilized, frozen, or washed fecal material), delivery routes (oral capsules, nasojejunal, colonoscopic, or transendoscopic enteral), and dosing schedules. Several studies incorporated preparatory steps such as bowel cleansing and antibiotic priming, although protocols were heterogeneous. Outcomes focused on GI symptoms assessed by the Bristol Stool Form Scale (BSFS) and the Gastrointestinal Symptom Rating Scale (GSRS), alongside ASD-related behaviors measured using validated scales including the Aberrant Behavior Checklist (ABC), Childhood Autism Rating Scale (CARS), Social Responsiveness Scale (SRS), and Vineland Adaptive Behavior Scales (VABS). Safety endpoints included reported adverse events.
Key Findings
Most before-and-after studies reported substantial improvements in both GI and behavioral symptoms after FMT, with reductions in stool irregularities, constipation rates, and GSRS scores demonstrating clinically meaningful GI symptom relief. Behavioral improvements were reflected by decreased scores on ABC and CARS and enhanced social functioning via SRS and VABS. Notably, Kang et al. documented sustained symptom amelioration and microbial diversity increases persisting up to two years post-treatment.
RCTs yielded mixed results. Wang et al. observed significant GI symptom improvement in the FMT group compared to placebo, with concomitant behavioral enhancements on CARS and SRS but not on ABC. In contrast, Wan et al. found no significant differences in ASD symptom scores between FMT and placebo cohorts, though a subgroup with higher donor microbiota engraftment showed better adaptive social outcomes. Both RCTs confirmed FMT safety, reporting only mild, transient adverse events akin to placebo.
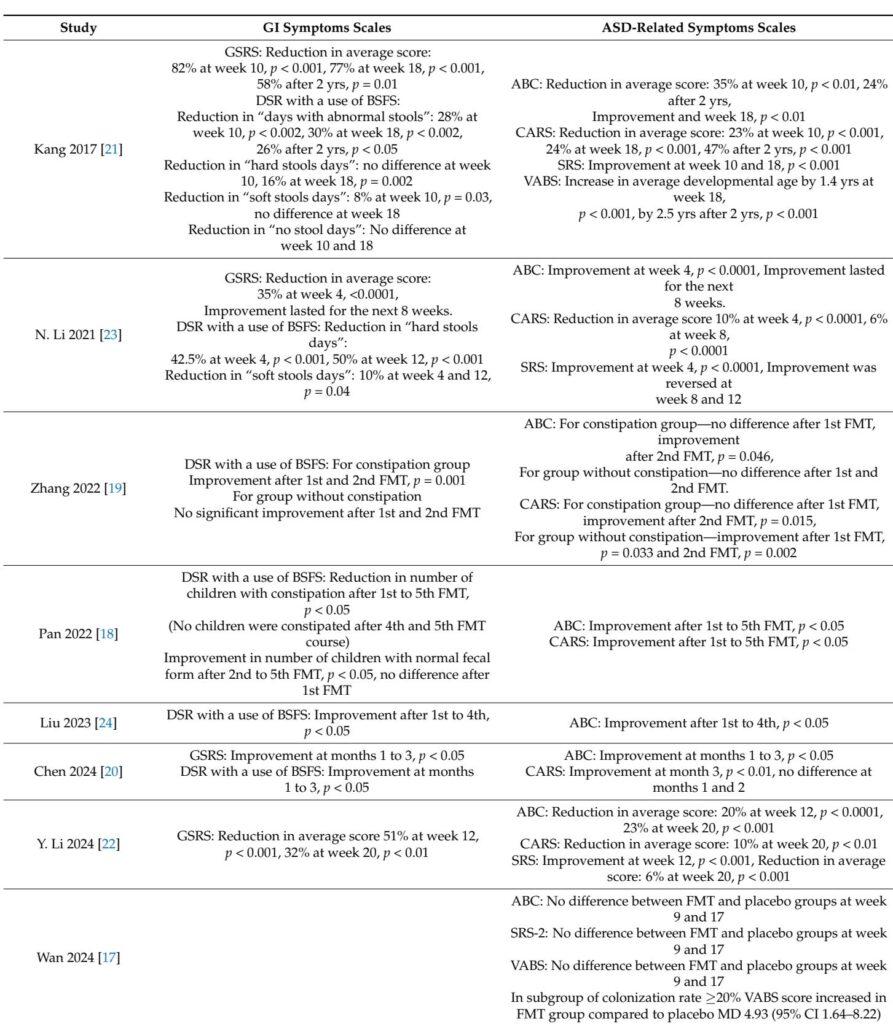
Microbiota analyses across studies highlighted increased alpha diversity and beneficial shifts toward profiles rich in Bifidobacterium, Prevotella, and other taxa linked to gut health. Associations between microbial composition and metabolic markers, including short-chain fatty acids and neurotransmitter metabolites, suggested mechanisms mediating FMT’s effects on neurobehavior.
Despite promising signals, limitations included small sample sizes, heterogeneous protocols, reliance on parent-reported outcome measures prone to bias, and short follow-up durations in most studies. The lack of standardized FMT preparation and delivery methods, alongside variable use of preparatory antibiotics or bowel cleansing, complicates direct comparison and generalizability.
Expert Commentary
The gut–brain axis provides a biologically plausible framework for FMT’s therapeutic rationale in ASD, where restoring microbial balance may attenuate neuroinflammatory processes and improve gut barrier integrity, influencing behavioral symptoms. However, methodological constraints and inconsistent RCT findings temper enthusiasm and underscore the critical need for optimized, standardized clinical trial designs. Future investigations should prioritize objective clinician-administered behavioral assessments, such as the Autism Diagnostic Observation Schedule, and extend follow-up to assess durability. Moreover, clarifying the role of preparatory regimens like bowel cleansing and antibiotics is vital, as emerging evidence from other clinical contexts suggests these may impact microbiota engraftment and treatment success. Concurrent evaluation of dietary factors and habitual microbiota-modulating exposures is warranted to mitigate confounding.
Conclusion
Current evidence indicates that FMT is a safe intervention that may improve gastrointestinal and behavioral symptoms in children and adolescents with ASD. Although observational studies and some RCT data suggest benefit, definitive conclusions are limited by study quality and variability. Robust, adequately powered, placebo-controlled RCTs with standardized protocols and comprehensive outcome measures are essential to validate FMT’s efficacy, elucidate mechanisms, and guide clinical implementation. Establishing consensus on optimal FMT preparation, delivery, and dosing will be key to advancing this promising microbiome-based therapy toward improving the lives of individuals with ASD.
Reference
Liber A, Więch M. The Impact of Fecal Microbiota Transplantation on Gastrointestinal and Behavioral Symptoms in Children and Adolescents with Autism Spectrum Disorder: A Systematic Review. Nutrients. 2025 Jul 7;17(13):2250. doi: 10.3390/nu17132250. PMID: 40647353; PMCID: PMC12252074.