Highlights
- Clarifies the definitions and clinical significance of major oncology trial endpoints: OS, DFS, PFS, ORR, CR, PR, SD, PD, and DCR.
- Offers practical guidance for interpreting these endpoints in both clinical practice and research settings.
- Discusses the advantages, limitations, and appropriate application of each endpoint within different trial designs.
Background and Clinical Context
Clinical trials in oncology hinge on well-defined endpoints to measure the efficacy of novel therapeutics. As cancer therapies become more diverse and personalized, understanding these endpoints has become essential for clinicians, investigators, and regulators alike. Endpoints such as Overall Survival (OS), Disease-Free Survival (DFS), Progression-Free Survival (PFS), Objective Response Rate (ORR), Complete Response (CR), Partial Response (PR), Stable Disease (SD), Progressive Disease (PD), and Disease Control Rate (DCR) are now central to evaluating clinical benefit, regulatory approval, and patient guidance.
The rising global cancer burden underscores the need for rigorous, interpretable endpoints. According to GLOBOCAN 2020, cancer is a leading cause of morbidity and mortality worldwide, necessitating accurate efficacy metrics for new drugs to ensure meaningful clinical impact [1].
Study Design Considerations: Endpoints in Oncology Trials
Oncology trials are commonly randomized controlled trials (RCTs) or single-arm studies, each with distinct endpoint strategies. The choice of endpoints depends on disease stage, expected outcomes, patient population, and regulatory guidance. The “Guidelines for Statistical Design of Antitumor Drug Clinical Trials” by China’s Center for Drug Evaluation echo international standards such as those from the U.S. FDA and EMA.
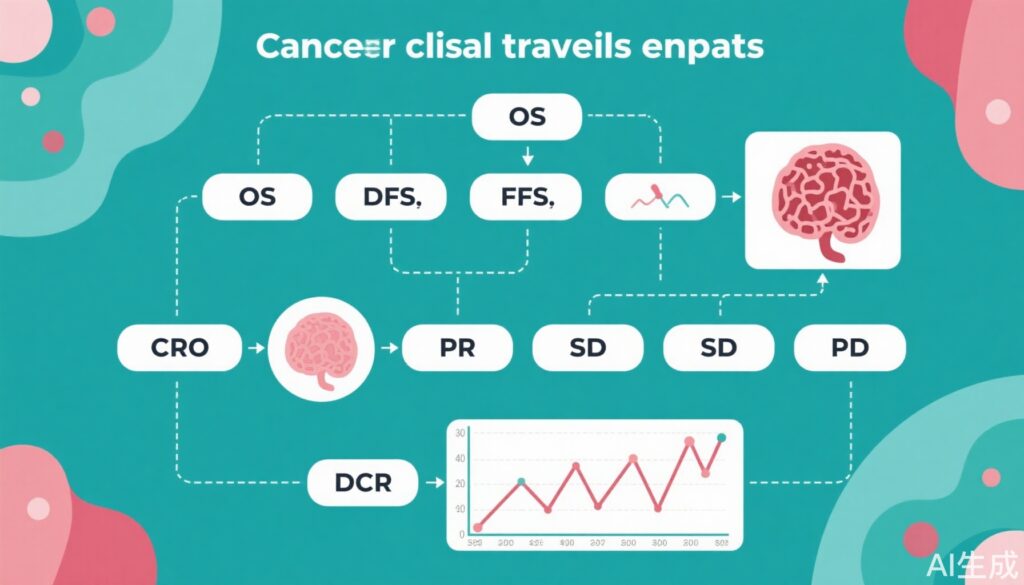
– **OS (Overall Survival):** Defined as the time from randomization (or treatment initiation in single-arm studies) to death from any cause.
– **DFS (Disease-Free Survival):** Time from randomization to disease recurrence or death, used primarily in adjuvant (post-surgical/radiation) settings.
– **PFS (Progression-Free Survival):** Time from randomization to documented tumor progression or death, often used in advanced/metastatic settings.
– **ORR (Objective Response Rate):** The proportion of patients achieving tumor shrinkage (CR or PR) per standardized criteria (e.g., RECIST 1.1 for solid tumors).
– **CR (Complete Response):** Disappearance of all target lesions and normalization of tumor markers for a minimum duration (usually ≥4 weeks).
– **PR (Partial Response):** ≥30% reduction in the sum of diameters of target lesions, maintained for at least 4 weeks.
– **SD (Stable Disease):** Insufficient shrinkage for PR and insufficient increase for PD.
– **PD (Progressive Disease):** ≥20% increase in the sum of target lesion diameters or appearance of new lesions.
– **DCR (Disease Control Rate):** Proportion of patients with CR, PR, or SD maintained beyond a minimum duration.
Key Findings and Clinical Interpretation
1. Overall Survival (OS): OS is the gold standard for assessing clinical benefit due to its objectivity and precision. It is the endpoint least subject to measurement bias and is universally interpretable. However, OS requires long-term follow-up and large sample sizes, and its utility may be confounded by subsequent lines of therapy or crossover treatments. Comparisons of OS across different studies or single-arm studies are unreliable due to inter-trial heterogeneity.
2. Disease-Free Survival (DFS) & Progression-Free Survival (PFS): DFS and PFS serve as important surrogate endpoints, especially when OS is not feasible due to prolonged survival or ethical constraints. DFS is mainly used in the adjuvant setting (e.g., post-surgery), indicating time until recurrence or death. PFS is more common in advanced/metastatic disease, reflecting control over tumor progression. While PFS can be measured earlier than OS, it is susceptible to assessment bias, especially in open-label or single-arm trials. Control arms are necessary for meaningful interpretation. Imaging data must be meticulously archived for audit and verification, and attention to censoring and missing data is critical for statistical validity [2].
3. Objective Response Rate (ORR), Complete Response (CR), and Partial Response (PR): ORR, calculated as the sum of CR and PR, is a direct measure of tumor shrinkage and is widely used in early-phase and accelerated approval settings. However, ORR alone does not capture the duration or quality of the response. For drugs with high or unprecedented ORRs, especially in refractory or rare cancers, regulatory agencies may grant conditional approvals pending confirmation of benefit in PFS or OS. CR and PR are clearly defined by RECIST or similar criteria and must persist for a minimum duration (≥4 weeks) to be counted.
4. Disease Control Rate (DCR), Stable Disease (SD), and Progressive Disease (PD): DCR extends response evaluation by including patients with sustained SD, recognizing that disease stabilization itself may confer clinically meaningful benefit, particularly for cytostatic agents. SD is defined as a lack of sufficient change to meet PR or PD criteria, while PD denotes unequivocal disease worsening. DCR is particularly relevant in settings where tumor shrinkage is infrequent but disease stabilization is common. The calculation: DCR = CR + PR + SD (meeting the minimum duration), while ORR = CR + PR.
Expert Commentary
Selection and interpretation of endpoints must be tailored to disease context, therapeutic mechanism, and patient needs. OS remains the most definitive efficacy measure, but regulatory and clinical practice increasingly recognize the value of surrogate endpoints like PFS and ORR, especially in settings of high unmet need or rapid drug development. The limitations of each endpoint—such as PFS’s vulnerability to assessment bias or ORR’s inability to reflect durability—underscore the importance of comprehensive endpoint reporting, including response duration and time to response.
Guideline bodies, including the U.S. FDA, EMA, and China CDE, recommend that trials preserve imaging and source data, predefine assessment intervals, and clearly document criteria for progression and response. Ultimately, the integration of multiple endpoints, contextualized by the specific cancer type and patient population, provides the most robust assessment of clinical benefit.
Conclusion
Mastering the definitions, strengths, and limitations of OS, DFS, PFS, ORR, CR, PR, SD, PD, and DCR is essential for accurate interpretation of oncology clinical trials and informed decision-making. These endpoints, when appropriately selected and interpreted, enable clinicians and researchers to translate trial findings into real-world benefit for patients with cancer.
References
[1] Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71(3):209-249.
[2] Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228-247.