Highlight
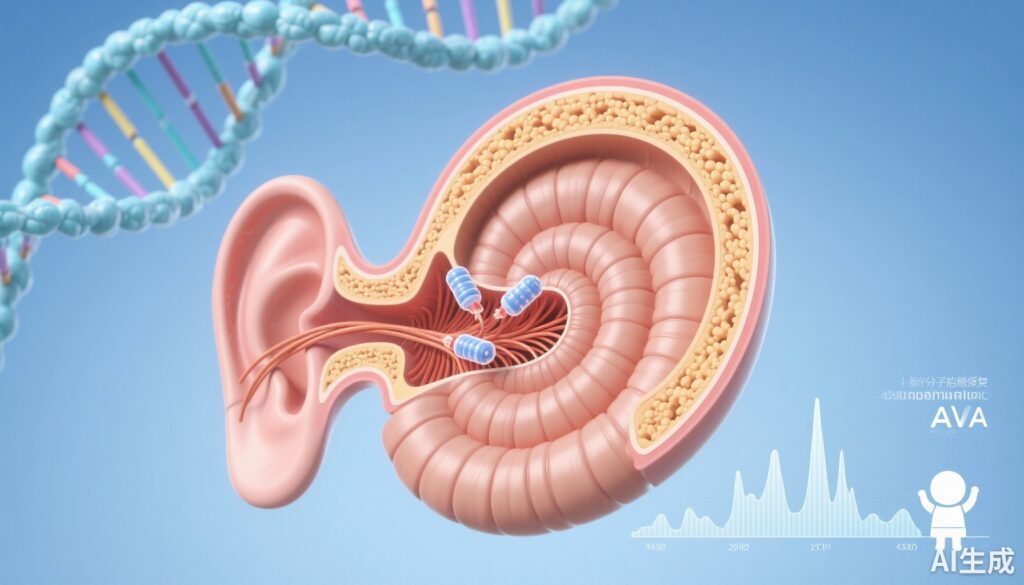
DB-OTO, a dual-AAV1 gene therapy using a hair-cell-specific synthetic promoter (mMyo15), reconstitutes full-length human OTOF variant 5 in inner hair cells and produces dose-dependent, durable recovery of auditory brainstem responses (ABRs) in otoferlin-deficient mice for at least 3 months. Hair-cell-restricted expression was critical for safety, and these preclinical data supported progression to a pediatric Phase I/II clinical trial.
Background: clinical context and unmet need
Biallelic loss-of-function variants in OTOF (encoding otoferlin) cause DFNB9, a non-syndromic congenital auditory neuropathy characterized by absent or severely reduced synchronous synaptic transmission from inner hair cells (IHCs) to spiral ganglion neurons despite preserved otoacoustic emissions in many cases. Affected infants typically present with severe-to-profound congenital or early-onset hearing loss and fail to develop spoken language without intervention. Conventional management includes cochlear implantation, which can be highly effective but requires surgery and lifelong device dependence; it also may not fully replicate natural sound encoding for complex listening tasks. There is therefore strong rationale for molecular therapies that restore the missing presynaptic protein and potentially preserve native cochlear architecture and neural coding.
Developing gene therapy for OTOF presents specific challenges. Otoferlin is a large multi-C2-domain protein (multiple exons encoding a >6 kb coding sequence), exceeding the packaging capacity of a single adeno-associated virus (AAV) vector. Delivery must reach IHCs efficiently and safely, and expression needs to be appropriately cell-restricted to avoid ectopic otoferlin expression that could be deleterious. Preclinical proof-of-concept in small-animal models has been essential to evaluate both functional rescue and safety before human translation.
Study design and methods
The study by Chung and colleagues (Mol Ther Methods Clin Dev. 2025) evaluated DB-OTO, a dual-AAV1 gene therapy composed of two vectors that together reconstitute a full-length human OTOF variant 5 (hOTOFv5) expression cassette. A synthetic hair-cell-specific promoter (mMyo15), engineered from murine Myosin 15a regulatory elements and sized ~1.0 kb, was used to drive restricted expression in IHCs. The authors selected this promoter after head-to-head comparisons of promoter constructs with a GFP reporter in ex vivo murine cochlear explants followed by in vivo validation in wild-type mice and cynomolgus macaques to assess transduction specificity and translation to nonhuman primates.
The therapeutic construct was delivered to neonatal Otof knockout mice via established inner-ear delivery routes. The study explored a >10-fold dose range to assess dose-dependency and safety. Primary functional endpoints included auditory brainstem responses (ABRs), which are standard electrophysiological measures of peripheral auditory pathway function in animal models. Molecular endpoints included immunohistochemical detection of otoferlin in IHCs and assessment of ectopic expression when using ubiquitous versus hair-cell-specific promoters. Durability was assessed out to at least three months post-injection.
Key findings
Promoter selection and specificity: Multiple promoter constructs were screened using GFP reporters in explant cultures. The 1.0 kb mMyo15 synthetic promoter achieved robust, high-level expression in inner hair cells with minimal off-target expression compared with ubiquitous promoters. In vivo validation in wild-type mice and cynomolgus macaques showed hair-cell-restricted transgene expression, supporting translational potential of the promoter design.
Reconstitution of full-length OTOF: The dual-AAV1 approach successfully reconstituted a functional hOTOFv5 expression cassette in hair cells. The two-vector system allowed delivery of a coding sequence larger than a single AAV capacity by restoring an intact expression cassette within transduced hair cells.
Functional recovery: DB-OTO produced dose-dependent recovery of ABR thresholds in otoferlin-deficient mice. At therapeutic doses within the evaluated range, treated animals showed establishment of ABRs indicative of restored synaptic transmission from IHCs. The magnitude of rescue correlated with dose and with the degree of otoferlin expression observed in IHCs.
Durability: Functional ABR recovery and otoferlin expression in IHCs were sustained for at least three months across the tested dose range. This sustained effect argues for a durable biological impact in the mouse model, although longer follow-up and evaluation in larger species are required to confirm persistence.
Safety and promoter importance: A key safety finding was that hair-cell–specific expression was critical. Comparison with a ubiquitous promoter driving hOTOFv5 expression led to safety concerns, implying that ectopic expression outside IHCs may be deleterious and that cell restriction mitigates off-target effects. No off-target pathology or major adverse effects were reported in the treated cohorts within the observation window.
Translational validation: The mMyo15 promoter and AAV1 capsid produced hair-cell–targeted transduction in cynomolgus macaques, lending supportive data for translation to human trials.
Interpretation and mechanistic insights
Otoferlin is believed to act as a calcium sensor and membrane-fusion facilitator at the inner hair cell synapse, supporting rapid and sustained synaptic vesicle replenishment required for high-fidelity auditory signaling. Restoring otoferlin expression in IHCs should therefore re-establish synchronous neurotransmitter release and improve neural responses to sounds. The present work demonstrates this mechanistic premise: molecular restoration of otoferlin in IHCs correlated with electrophysiological evidence of synaptic function recovery.
The dual-AAV approach addresses a critical technical barrier—the large size of the OTOF coding sequence—by delivering the therapeutic cassette in two parts that recombine or reconstitute intracellularly. The success of such strategies depends on co-transduction efficiency of target cells and accurate reconstitution, both of which appear adequate in the neonatal mouse inner ear at the doses tested.
Clinical and translational considerations
These preclinical results supported initiation of a Phase I/II clinical trial of DB-OTO in pediatric patients with OTOF-related hearing loss. Several translational aspects merit emphasis for clinicians and trialists:
- Window for intervention: Early treatment is likely advantageous because auditory pathways and language development depend on early sensory input. Prelingual patients identified by newborn screening may represent optimal candidates.
- Delivery route and anaesthesia: Intracochlear or round window administration under surgical conditions will be required in humans; perioperative safety and anesthesia in infants require careful planning.
- Immune considerations: Pre-existing or treatment-induced immunity to AAV1 capsids could impact efficacy and repeat dosing. Screening and management plans should be part of trial design.
- Outcome measures: While ABR is a robust electrophysiological endpoint, clinical trials should include comprehensive audiometric assessments (behavioral audiometry as age permits), speech and language outcomes, and patient- or parent-reported functional measures to capture meaningful benefit.
- Durability and scaling: Mouse longevity and immune responses differ from humans; longer-term follow-up and nonhuman primate data will inform durability and safety in the clinical setting.
Limitations and outstanding questions
Several caveats limit immediate extrapolation of the mouse data to human clinical benefit. First, co-transduction efficiency and reconstitution rates in human IHCs remain to be fully characterized; differences in inner ear size and architecture may influence vector distribution. Second, three-month durability in mice, while encouraging, is a limited timescale relative to lifelong hearing needs. Third, safety over longer periods, including potential ectopic expression or vector-related immunopathology, requires ongoing monitoring. Finally, functional recovery in mice was assessed primarily by ABR; the extent to which reconstituted otoferlin restores complex auditory processing, binaural hearing, and language outcomes in infants is unknown and must be established clinically.
Expert commentary
The study represents a thoughtful translational progression: rational promoter engineering to achieve hair-cell specificity, pragmatic dual-vector design to overcome size constraints, and cross-species validation including nonhuman primates. For clinicians caring for children with DFNB9, these data are the most direct preclinical evidence to date that genetic replacement of a large presynaptic protein can restore peripheral auditory physiology. The emphasis on hair-cell-specific expression answers a practical safety question that is often underappreciated in gene-therapy development.
That said, clinicians should counsel families that early-phase trials primarily evaluate safety and biological efficacy; restoration of spoken language and real-world hearing function will be revealed only by longitudinal clinical studies incorporating developmental endpoints. Cochlear implantation remains an evidence-based option with predictable outcomes and should be part of shared decision-making discussions.
Conclusion and future directions
DB-OTO demonstrates proof-of-principle that dual-AAV delivery of full-length hOTOFv5, when combined with a hair-cell-specific promoter, can restore and sustain auditory physiology in otoferlin-deficient mice. Hair-cell-targeted expression mitigates safety concerns associated with ubiquitous transgene expression. These preclinical data underpin an ongoing Phase I/II clinical trial in pediatric patients with OTOF-related deafness and represent an important step toward gene-based restoration of native cochlear function.
Key future priorities include longer-term safety and efficacy monitoring in clinical cohorts, optimization of delivery approaches for human cochlear anatomy, assessment of real-world auditory and language outcomes, and strategies to address pre-existing anti-AAV immunity. If clinical trials demonstrate meaningful benefit and acceptable safety, DB-OTO or similar approaches could expand treatment options for children with congenital auditory synaptopathy, potentially reducing reliance on prosthetic devices and improving developmental outcomes.
Funding and clinicaltrials.gov
Chung et al. acknowledged institutional and sponsor support as described in their paper. The study’s outcomes directly informed initiation of a Phase I/II trial; clinicians and families should consult the clinicaltrials.gov registry and trial-specific materials for registration identifiers and up-to-date enrollment information.
References
1. Chung Y, Koehler SD, Cancelarich S, et al. Functional, sustained recovery of hearing in Otoferlin-deficient mice using DB-OTO, a hair-cell-specific AAV-based gene therapy. Mol Ther Methods Clin Dev. 2025 Aug 25;33(4):101577. doi: 10.1016/j.omtm.2025.101577. PMID: 41036103; PMCID: PMC12481892.
2. Varga R, Jennings B, Hibbard L, et al. Mutations in OTOF, encoding otoferlin, cause DFNB9, a recessive form of nonsyndromic deafness. Nat Genet. 2003;35(3):258–264. (Foundational identification of OTOF as a cause of human congenital deafness.)
3. World Health Organization. Deafness and hearing loss. WHO fact sheet. Updated 2021. https://www.who.int/news-room/fact-sheets/detail/deafness-and-hearing-loss (accessed 2025).