Introduction
Effective glycaemic control in type 1 diabetes requires precise insulin management to reduce the risks of both hyperglycaemia and hypoglycaemia. Sodium–glucose cotransporter 2 (SGLT2) inhibitors, such as dapagliflozin, have emerged as adjunctive therapies that lower blood glucose through insulin-independent renal glucose excretion. Despite demonstrated benefits including improved postprandial glucose levels, blood pressure reduction, and weight loss, their use is tempered by an increased risk of diabetic ketoacidosis (DKA). The interplay of hormones such as glucagon-like peptide 1 (GLP-1), glucagon, and somatostatin is critical in glucose homeostasis and ketogenesis; however, the impact of dapagliflozin on these hormones in type 1 diabetes remains unclear.
Study Design
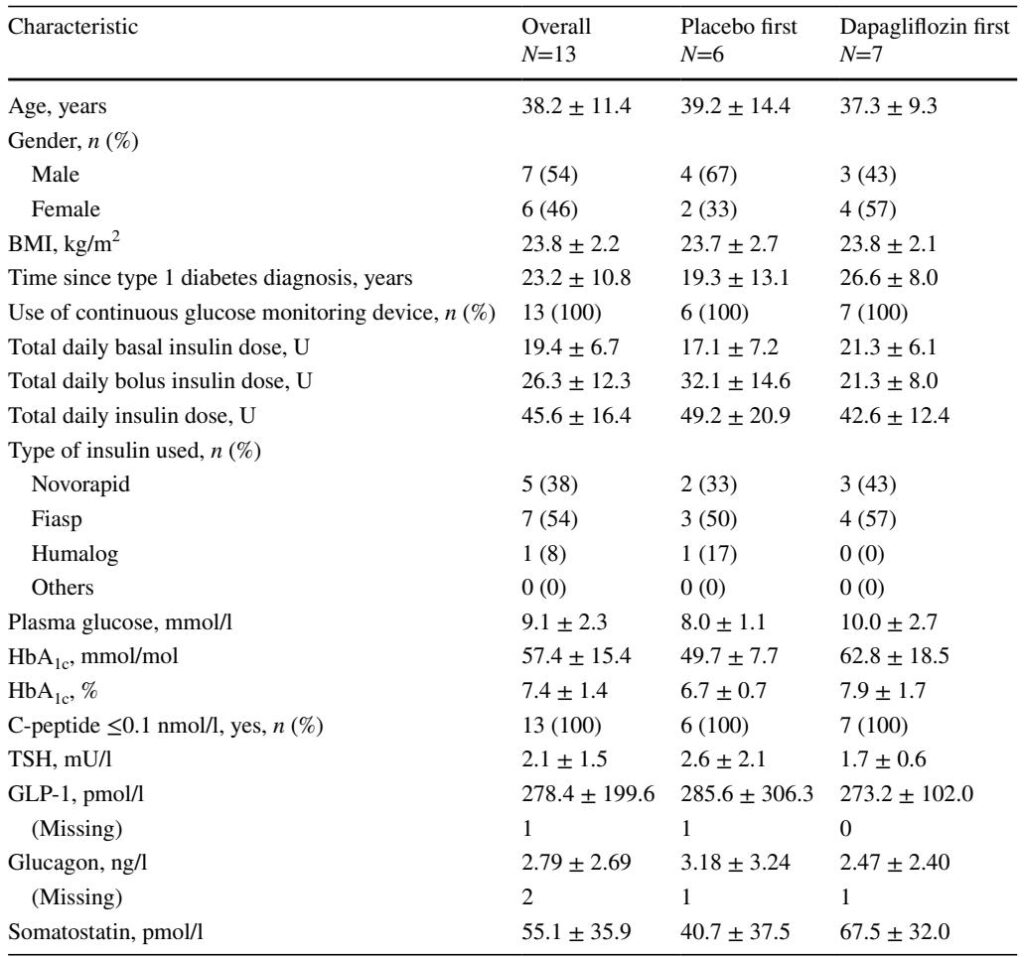
This randomised, placebo-controlled, open-label crossover study was conducted at the Department of Diabetes, Endocrinology, Clinical Nutrition & Metabolism, Inselspital, Bern University Hospital. Thirteen adults with type 1 diabetes (C-peptide 5 years, BMI 20–29 kg/m²) received 10 mg dapagliflozin or placebo daily for 7 days in two separate periods, with a 14-day washout in between. On day 7 of each period, participants underwent hyperinsulinaemic–euglycaemic clamps (HECs) and oral glucose tolerance test clamps (OGTTCs) measuring plasma GLP-1, glucagon, somatostatin, and ketone bodies. The primary endpoint was GLP-1 concentration during OGTTC; secondary endpoints included GLP-1 during HEC and glucagon, somatostatin, and ketone bodies during both clamp procedures. The study was unblinded, with randomisation performed electronically via REDCap, and all participants completed the protocol per study design.
Key Findings
Plasma GLP-1 concentrations did not differ significantly between dapagliflozin and placebo during either OGTTC (median 192.8 vs 176.3 pmol/l, p=0.7) or HEC (median 208.6 vs 203.1 pmol/l, p=0.7). Similarly, glucagon levels were comparable between treatments during both OGTTC (median 1.54 vs 1.54 ng/l, p=0.8) and HEC (median 1.59 vs 1.63 ng/l, p=0.3). Somatostatin concentrations also showed no significant differences between dapagliflozin and placebo in either clamp condition. However, plasma ketone bodies were significantly elevated after dapagliflozin treatment compared with placebo during OGTTC (median 0.10 vs 0.03 mmol/l, p<0.001) and HEC (median 0.15 vs 0.03 mmol/l, p<0.001). Moreover, exploratory analyses demonstrated significantly lower plasma glucose during OGTTC with dapagliflozin (7.84 vs 8.53 mmol/l, p<0.001), indicating improved glycaemic control. Importantly, no severe adverse events or episodes of diabetic ketoacidosis occurred, though mild, asymptomatic ketone elevations were recorded. These findings were consistent across per-protocol analyses and showed no carryover effects.
Expert Commentary
Contrary to earlier pilot data and studies in type 2 diabetes suggesting increased GLP-1 secretion with SGLT2 inhibition, this trial found no effect on incretin or glucagon secretion in type 1 diabetes. This is likely due to the lack of endogenous beta-cell function impacting enteroinsular hormonal signaling. The absence of glucagon increase contrasts with some reports indicating enhanced alpha-cell glucagon release from SGLT2 inhibition, possibly reflecting the difference between in vitro and in vivo drug concentrations or physiological complexity. Elevated ketones despite unchanged glucagon imply ketogenesis stimulation through mechanisms independent of glucagon, potentially involving insulin dose reductions, augmented lipolysis, or decreased renal ketone clearance. The modest ketone elevation without clinical ketoacidosis stresses the importance of patient selection and close monitoring during dapagliflozin use. Reduced plasma glucose and likely lowered insulin requirements confirm the insulin-sparing benefits of dapagliflozin in type 1 diabetes management.
Limitations
The open-label design introduces potential bias in participant behavior and outcome assessments, though the known ketone elevations likely resulted in partial unblinding despite any placebo effects. Nutritional intake was not standardized, which may affect GLP-1 variability. Blood glucose during clamps deviated slightly from strict euglycaemia but did not differ between groups. Plasma insulin levels were not measured, limiting insights into precise insulin-hormone interplay. The relatively small sample size restricts generalizability and precludes subgroup analyses such as gender effects. Longer-term outcomes and larger cohorts remain necessary to fully understand clinical implications.
Conclusion
Short-term adjunctive dapagliflozin therapy in adult type 1 diabetes patients increases ketone body production without influencing GLP-1, glucagon, or somatostatin secretion. While it improves glycaemic control and reduces plasma glucose levels, the associated ketogenesis highlights the elevated risk for diabetic ketoacidosis that requires careful clinical vigilance. These results underscore the necessity of personalized therapy, patient education, and ketone monitoring when integrating SGLT2 inhibitors into type 1 diabetes management. Further investigation into long-term hormonal effects and DKA mitigation strategies is warranted to optimize safety and efficacy.