Highlights
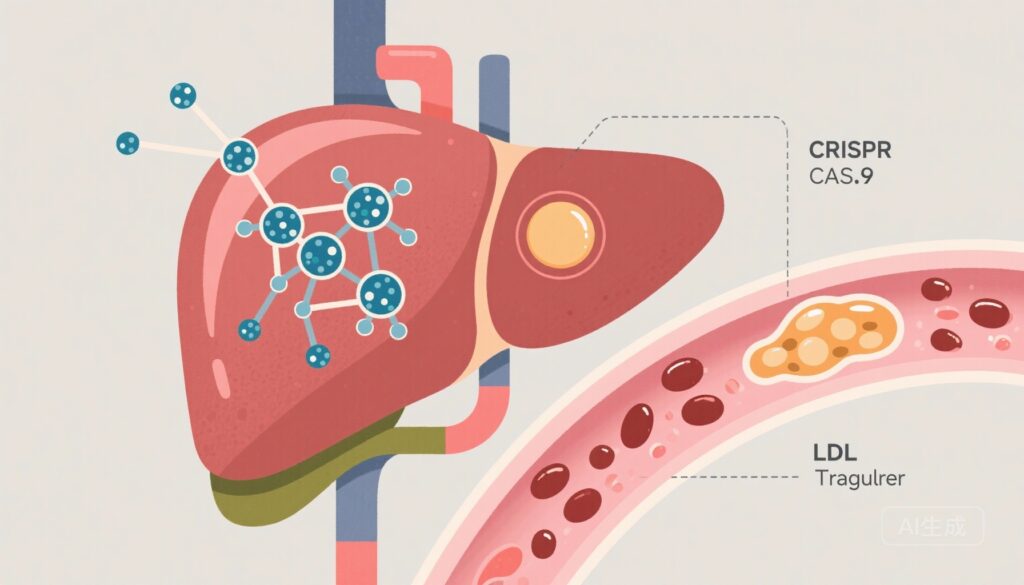
– CTX310, a lipid-nanoparticle (LNP) delivery of CRISPR-Cas9 mRNA and guide RNA targeting hepatic ANGPTL3, was administered as a single IV dose to 15 adults with refractory dyslipidemia in an ascending‑dose phase 1 trial.
– No dose‑limiting toxicities were attributed to CTX310. Dose‑dependent reductions in circulating ANGPTL3 were observed at doses ≥0.6 mg/kg, with mean reductions of ~33% at 0.6 mg/kg and ~74–80% at 0.7–0.8 mg/kg.
– Early safety signals included infusion reactions (20%), one transient transaminase elevation, two serious adverse events (one spinal disk herniation and one sudden death occurring on day 179 after the lowest dose), and the need for rigorous long‑term monitoring for off‑target effects and delayed adverse events.
Background
Angiopoietin‑like protein 3 (ANGPTL3) is a liver‑derived regulator of plasma lipid metabolism that inhibits lipoprotein lipase and endothelial lipase. Human loss‑of‑function variants in ANGPTL3 associate with reduced triglycerides, reduced LDL cholesterol, and lower lifetime risk of atherosclerotic cardiovascular disease, making ANGPTL3 an attractive therapeutic target for lipid lowering.
Therapeutic approaches to ANGPTL3 inhibition have included monoclonal antibodies and antisense oligonucleotides; these require repeated dosing. In contrast, in vivo somatic gene editing aims to create a durable loss of function with a single administration. Prior first‑in‑human in vivo CRISPR trials targeting transthyretin used LNP delivery of CRISPR‑Cas9 mRNA and reported dose‑dependent target knockdown and an acceptable short‑term safety profile, providing a precedent for this modality in the liver.
Study design
This open‑label, ascending‑dose phase 1 trial evaluated CTX310, an LNP‑encapsulated CRISPR‑Cas9 mRNA plus guide RNA directed to ANGPTL3, administered as a single intravenous infusion. Adults had uncontrolled hypercholesterolemia, hypertriglyceridemia, or mixed dyslipidemia despite maximally tolerated lipid‑lowering therapy. Cohorts received CTX310 at 0.1, 0.3, 0.6, 0.7, or 0.8 mg/kg. The primary endpoint was safety, including dose‑limiting toxicities. Secondary and exploratory endpoints included biochemical evidence of ANGPTL3 knockdown and related lipid effects; the report provides percent changes in ANGPTL3 levels by dose group. Full trial registration: Australia New Zealand Clinical Trials Registry ACTRN12623000809639. The study was funded by CRISPR Therapeutics and is reported by Laffin et al. in NEJM (2025).
Key findings
Fifteen participants received a single dose of CTX310 and had ≥60 days of follow‑up. Key results include:
- Safety: No dose‑limiting toxic effects attributed to CTX310 were reported. Serious adverse events occurred in two participants (13%): a spinal disk herniation (not temporally or mechanistically linked to the study drug in the report) and a sudden death at day 179 after receiving the 0.1 mg/kg dose. The cause of death was not specified in the summary; attribution to CTX310 was not established.
- Infusion reactions: Three participants (20%) experienced infusion‑related reactions. One participant with preexisting elevated aminotransferases had a transient rise to 3–5× baseline levels, peaking on day 4 and returning to baseline by day 14.
- On‑target biochemical effect (ANGPTL3 protein levels): A dose‑dependent reduction in circulating ANGPTL3 was observed. Mean percent changes from baseline in ANGPTL3 were: +9.6% (range −21.8 to 71.2) at 0.1 mg/kg; +9.4% (range −25.0 to 63.9) at 0.3 mg/kg; −32.7% (range −51.4 to −19.4) at 0.6 mg/kg; −79.7% (range −86.8 to −72.5) at 0.7 mg/kg; and −73.2% (range −89.0 to −66.9) at 0.8 mg/kg.
Notably, the lowest two dose cohorts (0.1 and 0.3 mg/kg) showed minimal or heterogeneous reductions in ANGPTL3, while doses ≥0.6 mg/kg produced consistent, substantial knockdown. The available report emphasizes protein reductions; detailed lipid endpoint data (LDL‑C, triglycerides, HDL‑C) and durability beyond initial follow‑up windows are not provided in the summary and will be essential to interpret clinical benefit.
Safety considerations and interpretation
The short‑term safety profile appears acceptable in this small cohort, with no acute dose‑limiting toxicities and manageable infusion reactions. However, several caveats are critical:
- Small sample size: Fifteen participants across five dose levels limit the ability to detect uncommon or delayed adverse events.
- Serious adverse events: Two SAEs were reported, including a sudden death occurring months after the lowest dose. Although causality was not established, such events require thorough investigation, adjudication, and longer follow‑up in larger cohorts.
- Hepatic safety: Transient transaminase elevation in one participant highlights the need for hepatic monitoring. The liver is the intended target organ, and hepatocyte injury, immune responses, or rare clonal expansions merit longitudinal surveillance.
- Immunologic and off‑target risks: LNPs and Cas9 protein/RNA can elicit innate and adaptive immune responses. Cas9‑induced double‑strand breaks (DSBs) carry risks of unintended large deletions, chromosomal rearrangements, or activation of p53 pathways; these biological outcomes require sensitive genomic assays (deep sequencing, unbiased off‑target detection, long‑read sequencing) and long‑term follow‑up for malignancy risk or clonal selection.
- Germline transmission: The systemic LNP approach preferentially targets hepatocytes; germline editing risk is theoretically low but must be excluded by biodistribution studies and reproductive safety precautions.
Mechanistic insights and comparison to other approaches
ANGPTL3 inhibition lowers triglycerides and LDL‑C in humans with loss‑of‑function alleles and in trials of monoclonal antibodies and antisense therapies. Unlike these approaches, CRISPR‑Cas9 nuclease editing aims to permanently disrupt ANGPTL3 in hepatocytes after a single dose. The observed dose‑dependent protein knockdown supports efficient hepatic delivery and on‑target editing at higher doses.
Prior in vivo CRISPR human trials (for transthyretin amyloidosis, using LNP‑delivered Cas9 mRNA and guide RNA) demonstrated similar dose‑dependent biochemical effects and an acceptable short‑term safety profile, lending translational plausibility to the CTX310 approach. However, nuance exists: nuclease‑based editing produces DSBs, and the spectrum of repair outcomes differs from base editing or prime editing technologies that avoid DSBs; these mechanistic differences have implications for safety and predictability of edits.
Limitations and unanswered questions
- Clinical efficacy: The report provides ANGPTL3 protein reductions but does not report detailed, systematic changes in LDL‑C, triglycerides, or cardiovascular outcomes. Whether the observed protein knockdown translates into clinically meaningful and durable lipid lowering remains to be shown.
- Durability and dose selection: The dose–response suggests a threshold effect at ~0.6 mg/kg; optimal dosing for maximal efficacy with acceptable safety requires larger, controlled studies and longer follow‑up to assess durability over years.
- Off‑target and genomic safety: The extent of on‑ and off‑target edits, large deletions, chromosomal rearrangements, or clonal expansions was not detailed in the summary; comprehensive genomic characterization is mandatory for regulatory evaluation.
- Generalisability: The trial included patients with refractory dyslipidemia on maximal therapy. Applicability to broader populations (e.g., patients at primary prevention risk, familial hypercholesterolemia) will depend on safety, efficacy, and risk–benefit assessments.
- Comparative effectiveness: How a one‑time gene‑editing intervention compares with established and emerging lipid‑lowering therapies (statins, PCSK9 inhibitors, ANGPTL3 monoclonal antibodies/antisense agents) in cost, access, safety, and durability is unknown.
Clinical implications and next steps
This first‑in‑human phase 1 experience with CTX310 demonstrates proof of principle that LNP‑delivered CRISPR‑Cas9 can reduce a hepatic protein target in humans with an acceptable short‑term safety profile at higher doses. The translational implications are substantial: a one‑time therapy that durably reduces atherogenic lipids could transform prevention strategies for high‑risk patients.
Key next steps include: expansion cohorts with the candidate optimal dose(s), robust lipid efficacy endpoints (LDL‑C, triglycerides), extended follow‑up for years to capture delayed adverse events, comprehensive unbiased off‑target genomic assessments, reproductive safety and biodistribution studies, and careful benefit–risk stratification for target populations. Head‑to‑head or comparative studies with other ANGPTL3 inhibitors and established lipid‑lowering agents will be needed to define the role of gene editing in clinical practice.
Conclusion
CTX310 produced dose‑dependent reductions in circulating ANGPTL3 protein in this small phase 1 study, with few immediate dose‑limiting toxicities reported. The findings provide early proof of concept for in vivo CRISPR‑Cas9 editing of a cardiometabolic target. However, the small sample size, limited follow‑up, and the occurrence of a late sudden death emphasize the need for larger, well‑controlled studies with long‑term safety surveillance and comprehensive genomic analyses before clinical application.
Funding and trial registration
The trial was funded by CRISPR Therapeutics. Australia New Zealand Clinical Trials Registry number: ACTRN12623000809639.
References
1. Laffin LJ, Nicholls SJ, Scott RS, Clifton PM, Baker J, Sarraju A, Singh S, Wang Q, Wolski K, Xu H, Nielsen J, Patel N, Duran JM, Nissen SE. Phase 1 Trial of CRISPR-Cas9 Gene Editing Targeting ANGPTL3. N Engl J Med. 2025 Nov 8. doi: 10.1056/NEJMoa2511778. Epub ahead of print. PMID: 41211945.
2. Gillmore JD, Gane E, Taubel J, Kao J, Fontana M, Maitland A, et al. CRISPR–Cas9 In Vivo Gene Editing for Transthyretin Amyloidosis. N Engl J Med. 2021;385:493–502.