Highlights
- Three consecutive fecal microbiota transplantations (FMT) did not lead to significant improvement in hepatic steatosis or glucose tolerance in patients with metabolic dysfunction-associated steatotic liver disease (MASLD).
- High baseline gut microbiota diversity may limit the therapeutic potential of FMT in this population.
- No consistent donor microbiota engraftment or microbial signatures associated with clinical response were identified.
- Future trials should consider targeting patients with lower baseline microbiota diversity.
Study Background and Disease Burden
Metabolic dysfunction-associated steatotic liver disease (MASLD), previously classified under the umbrella of nonalcoholic fatty liver disease (NAFLD), represents a growing global health issue. MASLD is characterized by the accumulation of fat in the liver in conjunction with metabolic dysfunction, often leading to progressive liver injury, fibrosis, and increased cardiovascular risk. Despite its rising prevalence, effective pharmacologic therapies remain limited, and current strategies rely heavily on lifestyle modification.
The gut-liver axis has emerged as a potential therapeutic target, with accumulating evidence linking gut microbiota composition to MASLD pathogenesis and progression. Fecal microbiota transplantation (FMT), which involves the transfer of gut microbial communities from a healthy donor to a recipient, has been proposed as a means to restore microbial balance and potentially ameliorate metabolic and hepatic abnormalities in MASLD. However, clinical evidence for its efficacy remains sparse and inconclusive.
Study Design
This was a double-blind, randomized controlled trial conducted to evaluate the impact of consecutive FMT on hepatic steatosis and metabolic outcomes in MASLD. Twenty patients meeting diagnostic criteria for MASLD were randomized in a 1:1 ratio to receive either allogeneic (donor-derived) or autologous (self-derived) FMT.
– Intervention: FMT was administered at weeks 0, 3, and 6.
– Donor Source: Allogeneic FMT material was obtained from two healthy donors.
– Endpoints: Primary outcome was change in hepatic steatosis measured by magnetic resonance imaging-derived proton density fat fraction (MRI-PDFF) from baseline to week 12. Secondary outcomes included changes in glucose tolerance (oral glucose tolerance test), liver biochemistry, and gut microbiota composition and engraftment.
Key Findings
– Primary Endpoint: There was no statistically significant difference in the change in MRI-PDFF between the allogeneic and autologous FMT groups at week 12 (p = 0.50), indicating no appreciable reduction in hepatic fat content attributable to FMT.
– Secondary Endpoints: Measures of liver biochemistry (e.g., ALT, AST) and glucose tolerance also did not demonstrate significant changes between groups after three FMT sessions.
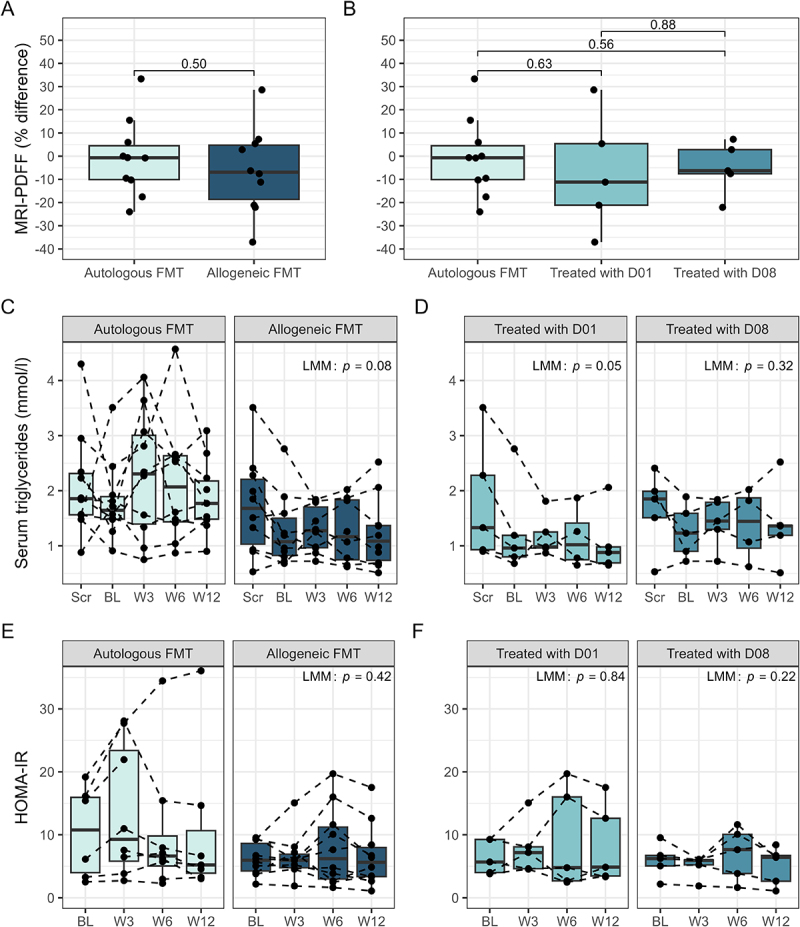
MASLD-associated clinical parameters over time. A, B: comparison of patient Δliver fat (%) (baseline to week 12), measured by magnetic resonance imaging-derived proton density fat fraction (MRI-PDFF), between intervention groups. C, D: serum triglycerides over time, separated by intervention group. E, F: homeostatic model assessment for insulin resistance (HOMA-IR) over time, separated by intervention group. Panels A and B show p-values from independent samples t-test and linear regression, respectively. Panels C-F show p-values from linear mixed effect models (LMM), comparing intervention groups over time (baseline to week 12). FMT: fecal microbiota transplantation; D01: donor one; D08: donor eight.
– Gut Microbiota Analysis:
– At baseline, patient stool microbiota exhibited high alpha diversity and similar composition across treatment arms.
– By week 12, microbiota profiles began to diverge (p = 0.02), but these changes were not clearly linked to treatment allocation or clinical response.
– Two bacterial taxa (families Gastranaerophilaceae and Rikenellaceae) were found to associate with post-FMT triglyceride levels, suggesting potential metabolic links, but no consistent microbial signatures were associated with FMT treatment or hepatic response.
– Donor microbiota engraftment was observed to be highly donor-specific but did not correlate with clinical efficacy.
– Safety: No significant adverse events related to FMT were reported over the 12-week study period, affirming the procedure’s safety profile in this patient population.
Expert Commentary
The findings from Groenewegen et al. provide important insights into the complexity of modifying gut microbiota in MASLD for therapeutic benefit. While preclinical and observational studies have highlighted the potential of FMT to modulate metabolic and hepatic pathways, this rigorously conducted RCT found no significant improvements in hepatic steatosis, metabolic markers, or liver biochemistry after three consecutive FMTs.
A notable aspect is the high baseline microbiota diversity in enrolled patients, which may have limited the potential for meaningful engraftment and therapeutic effect. Prior studies suggest that patients with lower microbial diversity or those with more disrupted gut microbiota may be more amenable to interventions like FMT. This trial’s negative findings underscore the need for improved patient stratification in future FMT studies for metabolic liver diseases.
Additionally, the lack of clear engraftment or consistent microbial signatures associated with response suggests that donor selection, engraftment dynamics, and host-microbiome interactions are critical variables warranting further investigation. The clinical safety of FMT was reaffirmed, but its role in MASLD management remains unproven.
Conclusion
In this double-blind, randomized controlled trial, consecutive FMT did not significantly impact hepatic steatosis, glucose tolerance, liver biochemistry, or gut microbiota signatures in patients with MASLD. These findings suggest that FMT, as currently implemented, may not offer clinical benefit for MASLD patients with high baseline microbiota diversity. Future studies should consider targeting patients with lower microbial diversity or more pronounced gut dysbiosis, and further explore the optimal donor selection and engraftment strategies. The search for effective, microbiota-based interventions in MASLD continues.
References
Groenewegen B, Ruissen MM, Crossette E, Menon R, Prince AL, Norman JM, Ballieux BEPB, Lamb HJ, Terveer EM, Keller JJ, Tushuizen ME. Consecutive fecal microbiota transplantation for metabolic dysfunction-associated steatotic liver disease: a randomized controlled trial. Gut Microbes. 2025 Dec;17(1):2541035. doi: 10.1080/19490976.2025.2541035 IF: 11.0 Q1