Highlights
- Population-based studies reveal specific thresholds of meaningful within-patient change on commonly used clinical outcome assessments (COAs) in early cognitive impairment.
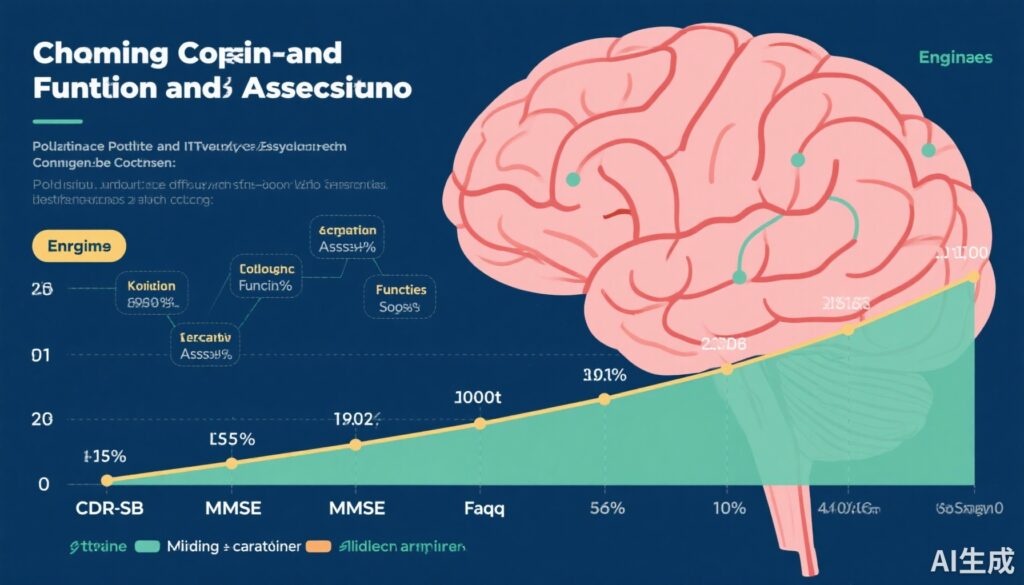
- Annualized change thresholds for CDR scale Sum of Boxes (0.49), MMSE (-1.01), and FAQ (1.04) in incident MCI offer benchmarks for interpreting clinical progression.
- These data enhance clinical trial planning and evaluation by anchoring COA changes to clinically meaningful functional and cognitive decline early in ADRD.
- Mechanistic insights into neurodegeneration underscore the importance of early detection and targeted interventions based on changes in cognition and function.
Background
Alzheimer’s disease and related dementias (ADRD) impose a substantial burden globally, with early detection and intervention increasingly recognized as vital for effective disease-modifying therapy (DMT) administration. Mild cognitive impairment (MCI) often represents the transitional clinical syndrome between normal aging and dementia, characterized by subtle cognitive decline without significant functional dependence. Clinical trials in ADRD increasingly utilize clinical outcome assessments (COAs) such as the Clinical Dementia Rating (CDR) scale Sum of Boxes (SB), Mini-Mental State Examination (MMSE), and Functional Activities Questionnaire (FAQ) to quantify cognitive and functional changes. However, establishing thresholds of within-patient change that are clinically meaningful, particularly early in disease progression, remains challenging. This gap complicates interpretation of trial outcomes and clinical decision-making. The Mayo Clinic Study of Aging (MCSA), a large population-based cohort, offers rigorous longitudinal data on cognitive aging and MCI. The recent work by Aakre et al. (2025) utilized incident MCI diagnosis as an anchor to estimate clinically meaningful annualized change thresholds on cognitive and functional COAs, thereby providing clinically interpretable benchmarks applicable to ADRD research and care.
Key Content
Population-Based Estimation of Clinically Meaningful Change Thresholds
Aakre et al. analyzed 646 MCSA participants aged 54–99 years, nearly evenly gender balanced (47% female), all of whom transitioned from cognitively unimpaired status to incident MCI during follow-up, with complete longitudinal COA data. Using incident MCI diagnosis as the anchor for clinical worsening, annualized change estimates (mean and 95% CI) were reported for three commonly used COAs:
- CDR scale Sum of Boxes (CDR-SB): +0.49 (0.43 – 0.55)
- Mini-Mental State Examination (MMSE): –1.01 (–1.12 to –0.91)
- Functional Activities Questionnaire (FAQ): +1.04 (0.82 – 1.26)
These thresholds represent the typical magnitude of within-patient change associated with progression to clinical MCI, indicating functionally and cognitively relevant decline across multiple assessment domains.
Clinical and Research Implications
The provision of data-driven, within-patient change benchmarks has multiple important implications:
- Trial Design and Interpretation: These thresholds can inform power calculations and anchor interpretation of outcome changes in clinical trials, improving detection of meaningful benefit of DMTs in early ADRD phases.
- Clinical Monitoring: Identification of patients crossing these thresholds can signal clinically meaningful decline, guiding treatment and care planning.
- Generalizability: Deriving estimates from a community-based sample enhances external validity beyond specialized clinic populations.
- Focus on Early Disease Course: Parameters address changes early in cognitive aging, a critical window for intervention before dementia onset.
Integration with Mechanistic and Therapeutic Advances in Alzheimer’s Disease
Understanding clinically meaningful change aligns with extensive research into ADRD pathophysiology and emerging therapeutics:
- Pathogenic processes: Neuroinflammation, mitochondrial dysfunction, amyloid-beta and tau pathology, synaptic lipid metabolism dysregulation, and genetic factors such as APOE genotypes contribute dynamically to early cognitive and functional decline. (Aakre et al.; referenced reviews on molecular pathways, neuroglobin, microglia; APOE genotype impact on neuropsychiatric symptoms)
- Biomarker innovation: Advanced electrochemical sensors for amyloid-β oligomers enhance early diagnosis, complementing clinical COA assessments.
- Therapeutic innovations: Immune interventions targeting amyloid and tau, mitochondrial-targeted therapies, and engineered immune cell strategies show promise, with clinical trial monitoring benefiting from accurate COA thresholds.
- Complex phenotyping: Integrative assessment combining clinical thresholds with biomarkers may improve individual patient stratification and therapeutic response prediction.
Expert Commentary
Clinicians and researchers require reliable, interpretable thresholds of change on COAs to differentiate normal aging from clinically meaningful progression indicative of underlying pathology. The MCSA-derived estimates by Aakre et al. represent a significant advance in defining these benchmarks within a real-world setting, addressing prior reliance on less generalizable cohorts or cross-sectional data. The use of incident MCI diagnosis as an anchor reinforces clinical relevance. However, the thresholds should be contextualized within individual variability, comorbidities, and concomitant biomarker data for holistic assessment. Future work should evaluate applicability across diverse populations and validate thresholds against longitudinal trajectories to dementia. Incorporation into clinical trial endpoints and real-world monitoring could enhance sensitivity and specificity for disease-modifying effects.
Furthermore, mechanistic insights from recent neurodegenerative disease research underscore the multifactorial landscape underpinning these clinical changes. Targeting mitochondrial function, neuroinflammatory pathways, and protein aggregation states alongside APOE genotype stratification may refine prognostication. Emerging biomarkers and novel therapeutic modalities necessitate dynamic integration with clinical evaluation tools such as CDR, MMSE, and FAQ, signaling a move toward personalized medicine in ADRD.
Conclusion
Clinically meaningful within-patient changes in cognitive and functional assessments early in Alzheimer’s disease and related dementias are crucial metrics to inform diagnosis, monitor progression, and evaluate therapeutic efficacy. The Mayo Clinic Study of Aging provides validated annualized change thresholds in CDR-SB, MMSE, and FAQ anchored to incident MCI diagnosis in a population-based sample. These benchmarks facilitate improved clinical trial design and real-world patient management. Integrative approaches combining clinical COAs with biomarkers and mechanistic understanding of neurodegeneration will further enhance disease interception strategies. Ongoing research should extend these findings to diverse cohorts and incorporate longitudinal validation to optimize precision in ADRD care.
References
- Aakre JA, Castillo AM, Graff-Radford J, Vemuri P, Machulda MM, Jack CR Jr, Knopman DS, Petersen RC, Vassilaki M. Clinically meaningful changes in cognitive and functional outcomes in a population-based study of cognitive aging. Alzheimers Dement (N Y). 2025 Sep 24;11(3):e70160. doi: 10.1002/trc2.70160.
- Luo Y, Yin B, Liu L, et al. Role of peroxisome proliferator-activated receptor alpha in neurodegenerative diseases and other neurological disorders: Clinical application prospects. Neural Regen Res. 2026 Apr 1;21(4):1468-1482. doi: 10.4103/NRR.NRR-D-24-01371.
- García-Osta A, et al. Recent advances in immunotherapy targeting amyloid-beta and tauopathies in Alzheimer’s disease. Neural Regen Res. 2026 Feb 1;21(2):577-587. doi: 10.4103/NRR.NRR-D-24-00846.
- Wang J, et al. Neuropsychiatric symptoms and apolipoprotein E genotypes in neurocognitive disorders. Neural Regen Res. 2026 Apr 1;21(4):1528-1541. doi: 10.4103/NRR.NRR-D-24-01274.
- Zhang Q, et al. Potential common pathogenesis of several neurodegenerative diseases. Neural Regen Res. 2026 Mar 1;21(3):972-988. doi: 10.4103/NRR.NRR-D-24-01054.