Highlights
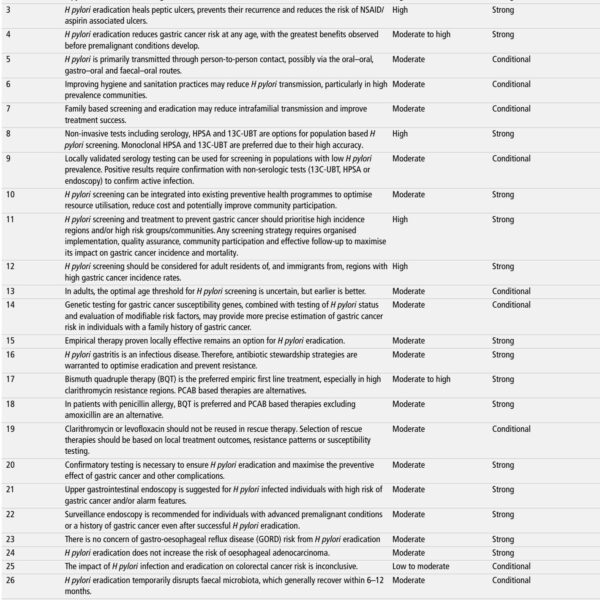
– In a multinational randomized trial (ANDROMEDA‑SHOCK‑2), personalized hemodynamic resuscitation targeting capillary refill time (CRT) improved a hierarchical composite of death, duration of organ support, and length of stay (win ratio 1.16, 95% CI 1.02–1.33; P = .04).
– The primary benefit was a shorter duration of vital support (vasoactives, mechanical ventilation, kidney replacement therapy); mortality differences were small and not the main driver.
– The CRT‑guided protocol integrates bedside assessments (CRT, pulse pressure, diastolic arterial pressure, fluid responsiveness, echocardiography) to individualize fluid, vasopressor, and inotrope use early in shock.
Background: unmet need in early septic shock resuscitation
Septic shock remains a leading cause of death in critically ill patients worldwide. Although the broad principles of early source control, antimicrobial therapy, and hemodynamic support are established, the optimal approach to hemodynamic resuscitation in the first hours of septic shock is unresolved. Early trials of fixed protocols (for example, early goal‑directed therapy) yielded conflicting results over two decades, highlighting heterogeneity in patient physiology and the potential importance of individualized approaches (Rivers et al., NEJM 2001; later multicenter trials showed mixed effects).
Capillary refill time (CRT) is a rapid bedside measure of peripheral perfusion; it is inexpensive, immediately available, and reflects microcirculatory and peripheral vasomotor status. Interest has grown in using CRT to guide resuscitation because it is physiologic, repeatable with training, and potentially responsive to fluids and vasoactive interventions. ANDROMEDA‑SHOCK‑2 tests whether a structured, CRT‑targeted, personalized protocol improves clinically meaningful outcomes when applied early in septic shock.
Study design and interventions
ANDROMEDA‑SHOCK‑2 is a pragmatic, international, randomized clinical trial conducted across 86 centers in 19 countries. Patients meeting criteria for septic shock within the first 4 hours were randomized to the CRT‑personalized hemodynamic resuscitation (CRT‑PHR) protocol (n = 720) or usual care (n = 747). Enrollment ran from March 2022 to April 2025 (last follow‑up July 2025).
The CRT‑PHR intervention used a stepwise bedside algorithm incorporating capillary refill time as the primary target, together with assessments of pulse pressure, diastolic arterial pressure, fluid responsiveness testing, and bedside echocardiography. These data were used to tailor decisions about intravenous fluids, vasopressor initiation and titration, and inotrope administration. Usual care followed local practice and institutional protocols without the structured CRT‑PHR algorithm. Randomization was stratified by the median APACHE II score at admission.
The prespecified primary outcome was a hierarchical composite at 28 days: first priority death, then duration of vital support (vasoactive therapy, mechanical ventilation, kidney replacement therapy), then length of hospital stay. The win ratio approach compared all possible pairs between groups using the hierarchy to determine winners. Secondary outcomes included 28‑day mortality, vital support‑free days, and hospital length of stay.
Key findings
Of 1,501 randomized patients, 1,467 were included in the primary analysis (mean age 66 ± 17 years; 43.3% female). Main results are summarized below.
Primary hierarchical composite
There were 131,131 wins (48.9%) favoring the CRT‑PHR group and 112,787 wins (42.1%) favoring usual care, yielding an overall win ratio of 1.16 (95% CI, 1.02–1.33; P = .04). The statistical approach compared every patient in the CRT arm with every patient in usual care, adjudicating pairwise winners based on the hierarchical outcomes.
Components of the composite
Pairwise wins attributed to each level of the hierarchy were distributed as follows (CRT‑PHR vs usual care):
- Death: 19.1% vs 17.8%
- Duration of vital support: 26.4% vs 21.1%
- Length of hospital stay: 3.4% vs 3.2%
These figures indicate that the dominant contributor to the composite benefit was a reduction in the duration of vital organ support in the CRT‑PHR arm. The mortality contribution to the win counts was smaller and did not represent a definitive mortality reduction signal.
Secondary outcomes and safety
Secondary outcomes reported included vital support‑free days and 28‑day mortality, but the primary composite was the principal inference. The trial report emphasizes the primary win‑ratio advantage driven by fewer days requiring organ support rather than a substantial mortality difference. The provided summary did not list unexpected safety signals; the full manuscript should be consulted for adverse event analyses and subgroup effects (including APACHE II strata, timing of therapy, and center‑level heterogeneity).
Interpretation and clinical significance
ANDROMEDA‑SHOCK‑2 provides evidence that an individualized, CRT‑guided hemodynamic strategy applied early in septic shock can reduce the duration of organ support and produce a modest improvement on a composite clinical endpoint. The win ratio of 1.16 indicates a statistically significant but clinically moderate advantage across the composite hierarchy.
Importantly, the principal benefit was fewer days on vasoactive drugs, mechanical ventilation, or renal replacement therapy. Shorter organ support has implications for ICU resource use, complications related to prolonged support, and patient recovery trajectories—even when absolute mortality differences are small.
Mechanistic plausibility
Targeting CRT prioritizes restoration of peripheral perfusion and microcirculatory flow. CRT is sensitive to vasomotor tone and local perfusion pressure; intervening to normalize CRT may signal improved tissue oxygenation and reduced progression to multi‑organ dysfunction. The CRT‑PHR algorithm’s integration of pulse pressure, diastolic arterial pressure, fluid responsiveness testing, and echocardiography allows clinicians to distinguish whether hypoperfusion reflects hypovolemia, vasodilation, or impaired cardiac output—facilitating tailored therapy rather than a one‑size‑fits‑all fluid load or vasopressor strategy.
Strengths of the trial
- Large, pragmatic, multinational randomized design across diverse healthcare settings enhances generalizability.
- Early enrollment (within 4 hours) captures the critical therapeutic window for hemodynamic interventions.
- The hierarchical outcome and win‑ratio method emphasize clinically relevant priorities (survival first, then organ support), aligning trial analysis with practical decision‑making.
- The CRT‑PHR protocol is physiologic and uses readily available bedside tools, potentially allowing wider adoption where trained personnel and ultrasound are available.
Limitations and caveats
- The win‑ratio composite is statistically novel for many clinicians and can be harder to intuitively translate into absolute risk reductions; clinicians should interpret effect sizes carefully.
- The overall effect size is modest; while statistically significant, the clinical importance depends on the weight placed on reducing organ support days versus mortality.
- Unblinded management could introduce bias for outcomes susceptible to clinician decision‑making (e.g., timing of liberation from ventilator, initiation/discontinuation of renal replacement therapy, or hospital discharge decisions).
- Implementation required trained clinicians able to perform and interpret bedside echocardiography and fluid responsiveness tests; feasibility in low‑resource settings may be limited.
- Heterogeneity across centers and patient subgroups may exist; the magnitude of benefit in specific populations (e.g., high vs low APACHE II strata) should be explored in subgroup reports.
Implications for practice and guidelines
The results support individualized, physiology‑driven resuscitation in early septic shock using CRT as a pragmatic target. For centers with training and capacity to perform the CRT‑PHR algorithm (including bedside ultrasound and fluid responsiveness testing), adopting a CRT‑guided approach can be considered to reduce the duration of organ support. However, widespread guideline changes will likely await corroborative data, cost–benefit analyses, and implementation studies that address training needs, protocol compliance, and reproducibility across healthcare contexts.
Clinicians should view CRT as an adjunct to, not a replacement for, comprehensive hemodynamic assessment and early sepsis care priorities (antibiotics, source control). Until integrated into guideline recommendations, CRT‑guided protocols may be most applicable in centers with established expertise in bedside hemodynamic assessment.
Expert commentary and next steps
ANDROMEDA‑SHOCK‑2 advances the evidence base supporting personalization of hemodynamic therapy in septic shock. The study aligns with a broader shift from static targets toward dynamic, patient‑specific goals. Future work should focus on: pooled analyses or meta‑analyses including earlier CRT trials to increase power for mortality endpoints; pragmatic implementation research assessing training pathways and fidelity; health‑economic analyses quantifying ICU resource savings from reduced organ support; and mechanistic studies linking CRT changes to microcirculatory and biomarker endpoints.
Conclusion
In patients with early septic shock, a personalized CRT‑targeted hemodynamic protocol produced a statistically significant benefit on a hierarchical composite outcome—primarily by shortening duration of vital organ support. The findings support the physiologic rationale for CRT‑guided resuscitation and suggest a practical, bedside approach to individualizing early hemodynamic therapy. Broader adoption should consider center capability, training, and need for confirmatory and implementation studies.
Funding and registration
Trial registration: ClinicalTrials.gov Identifier: NCT05057611. Funding details and full safety/adverse‑event reporting are provided in the primary JAMA report (ANDROMEDA‑SHOCK‑2 Investigators et al., JAMA 2025); readers should consult the full manuscript for sponsor and funding disclosures.
Selected references
1. ANDROMEDA‑SHOCK‑2 Investigators for the ANDROMEDA Research Network et al. Personalized Hemodynamic Resuscitation Targeting Capillary Refill Time in Early Septic Shock: The ANDROMEDA‑SHOCK‑2 Randomized Clinical Trial. JAMA. 2025 Oct 29:e2520402. doi: 10.1001/jama.2025.20402. PMID: 41159835; PMCID: PMC12573117.
2. Rivers E, et al. Early goal‑directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345(19):1368–1377.
3. Rhodes A, et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock 2021. Intensive Care Med. 2021;47(11):1181–1247.
Note
Clinicians should review the full trial publication for granular data, subgroup analyses, protocol details, and adverse events before changing local practice.