Highlights
– New multicenter cohort-derived basal serum calcitonin thresholds using modern electrochemiluminescence/chemiluminescence assays predict graded extents of lymph node metastasis (LNM) in medullary thyroid carcinoma (MTC).
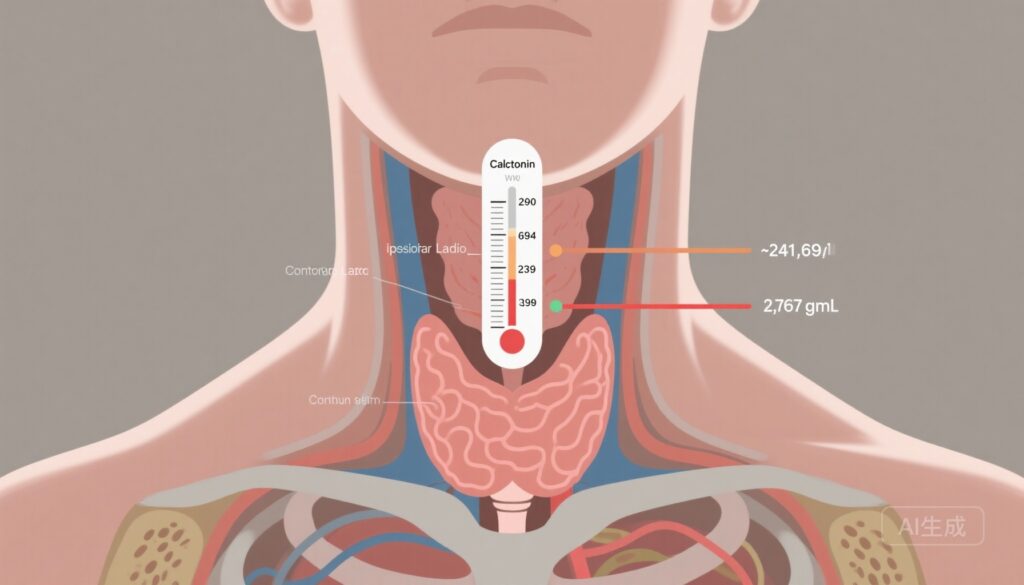
– Proposed cutoffs (241.9, 693.9, 2378.5, 2787.1 pg/mL) outperformed American Thyroid Association (ATA) guideline thresholds for predicting nodal compartments and discriminating structural recurrence-free survival (SRFS).
– These updated thresholds may inform biomarker-guided selective neck dissection algorithms but require prospective validation and consideration of assay-specific performance.
Background and disease burden
Medullary thyroid carcinoma (MTC) arises from parafollicular C cells and accounts for 3–5% of thyroid cancers. Unlike differentiated thyroid cancers, MTC secretes calcitonin—a highly sensitive and specific biomarker reflecting tumor burden. Lymph node metastasis (LNM) is common at diagnosis and is the major determinant of surgical planning and prognosis. Current management emphasizes total thyroidectomy with appropriate compartmental lymph node dissection. However, the optimal extent of neck dissection (central, lateral, and mediastinal compartments) remains contested when preoperative imaging is equivocal.
Guideline statements, including the 2015 American Thyroid Association (ATA) MTC guidelines, recognize preoperative basal calcitonin as a tool to estimate nodal disease risk and guide the extent of surgery. Historically published calcitonin thresholds were derived using older immunoassays (e.g., radioimmunoassays) and may not translate directly to modern electrochemiluminescent or chemiluminescent platforms. Given inter-assay variability and evolving analytic methods, updated, assay-relevant cutoffs are clinically important to refine selective neck dissection and avoid both undertreatment and overtreatment.
Study design
Du and colleagues performed a retrospective multicenter cohort study (13 hospitals in China) including 509 consecutively treated, initially managed MTC patients from 2011–2024 who had preoperative basal serum calcitonin measured by electrochemiluminescence or chemiluminescence assays. Patients were randomly split into a training cohort and a validation cohort (2:1). The primary exposure was preoperative basal calcitonin level; the primary outcomes were the extent of pathologic lymph node involvement (categorized as no LNM, central LNM, ipsilateral lateral LNM, bilateral/contralateral lateral LNM, and upper mediastinal LNM) and structural recurrence-free survival (SRFS). Analyses included determination of optimal calcitonin thresholds in the training set, assessment of discriminative performance versus ATA-recommended thresholds, and validation in the separate cohort. Follow-up median was 52 months (IQR 27–84).
Key findings
Population characteristics: Among 509 patients, median age was 50 years (IQR 40–59), 54.8% were female, and the median follow-up was 52 months. Patients were distributed across nodal categories with a clear positive correlation between basal calcitonin level and extent of nodal disease (effect size η2 = 0.28).
Derived calcitonin thresholds
Using the training cohort, investigators identified basal calcitonin thresholds associated with increasing burden and anatomical extent of nodal disease:
- Central compartment LNM: 241.9 pg/mL
- Ipsilateral lateral LNM: 693.9 pg/mL
- Bilateral and/or contralateral lateral LNM: 2378.5 pg/mL
- Upper mediastinal LNM: 2787.1 pg/mL
These thresholds were selected to discriminate progressively more extensive nodal involvement and were then applied to the validation cohort.
Performance compared with ATA thresholds
In both training and validation cohorts, the proposed thresholds demonstrated superior discrimination for the corresponding nodal disease extent compared with thresholds recommended in the 2015 ATA guidance. Performance metrics reported include improved sensitivity and specificity for predicting the anatomical compartment involved, and better separation of SRFS curves across groups partitioned by the new cutoffs.
Structural recurrence-free survival
Groups defined by the proposed thresholds showed stepwise differences in SRFS: higher basal calcitonin strata corresponded to greater risk of structural recurrence. The updated thresholds provided improved prognostic discrimination over the ATA-recommended cut points, suggesting not only diagnostic but also prognostic utility.
Secondary observations
The investigators also noted that assay type (electrochemiluminescence vs chemiluminescence) was contemporary and consistent across centers, supporting generalizability within modern analytic platforms. No major safety signals are relevant because the study is observational; however, implications pertain to the surgical management decisions that the thresholds would inform.
Expert commentary and clinical interpretation
This large multicenter cohort provides clinically actionable, assay-relevant calcitonin thresholds for stratifying risk of compartmental LNM in MTC. Several implications arise for practice:
- Refined preoperative risk stratification: The thresholds create a graduated risk model linking absolute calcitonin levels to likely nodal compartments involved. For example, a patient with basal calcitonin 693.9 pg/mL increase the likelihood of ipsilateral lateral disease.
- Guiding extent of neck dissection: When preoperative imaging is ambiguous, these thresholds could help decide whether to perform prophylactic central neck dissection alone versus adding ipsilateral lateral or bilateral lateral dissections, and when to consider upper mediastinal exploration.
- Prognostic stratification: The thresholds stratify SRFS and may therefore inform postoperative surveillance intensity and adjuvant discussions.
However, several caveats temper immediate clinical adoption:
- Retrospective design: As with all retrospective analyses, unmeasured confounding and selection biases may influence observed associations.
- Assay-specificity: The derived cutoffs are tied to electrochemiluminescent and chemiluminescent assays used across participating centers. Inter-laboratory differences, different platforms, and calibration variances mean thresholds may not be directly transferable to all settings without local calibration or conversion.
- Population generalizability: The cohort is from multiple Chinese centers; tumor biology, stage at presentation, and population-level factors may differ in other regions. External validation in diverse geographic cohorts is important.
- Surgical decision complexity: Calcitonin should inform but not replace clinical judgment. Cross-sectional imaging, ultrasound, fine-needle aspiration cytology, and patient comorbidity must be integrated into surgical planning.
Mechanistic plausibility is strong: calcitonin reflects tumor burden and secretory C-cell mass, which reasonably correlates with nodal tumor load. Prior studies have demonstrated associations between basal calcitonin and nodal disease, but assay evolution mandated recalibration of cutoffs. The study by Du et al. appropriately updates thresholds using modern immunochemiluminescent platforms.
Limitations and research gaps
Key limitations include retrospective design, potential heterogeneity in surgical practice and extent of lymph node sampling (which can bias nodal staging), and lack of direct head-to-head testing of thresholds on different commercial assay platforms. Prospective trials are needed to test whether biomarker-guided selective neck dissection based on these thresholds improves patient-centered outcomes (disease control, complications, quality of life) compared with imaging- or guideline-based strategies.
Clinical implications and recommendations
Clinicians should consider these updated thresholds as a useful adjunct to preoperative assessment in centers using electrochemiluminescence/chemiluminescence calcitonin assays. Practical steps include:
- Interpret preoperative basal calcitonin in the context of the assay type and local laboratory reference ranges.
- Use thresholds to inform shared decision-making about the likely extent of occult nodal disease and the benefits/risks of prophylactic compartmental dissections.
- Discuss the uncertainty and need for individualized decisions—especially when calcitonin is near one of the thresholds or when imaging is discordant.
- Advocate for prospective, ideally randomized, studies that test biomarker-guided surgical strategies against standard care with endpoints of recurrence, morbidity, and survival.
Conclusion
Du et al. present updated, assay-relevant basal calcitonin thresholds that stratify the anatomic extent of lymph node metastasis and better discriminate structural recurrence-free survival than prior guideline thresholds. These cutoffs have potential to refine preoperative risk stratification and guide selective neck dissection in MTC, but prospective validation across diverse assays and populations is necessary before broad implementation.
Funding and trials
The original study lists institutional and grant support as per the JAMA Otolaryngology publication. No clinicaltrials.gov identifier is provided because the work is observational and retrospective. Readers should consult the published article for detailed funding disclosures and the published erratum (Nov 13, 2025) noted in the citation.
References
1. Du Y, Shen C, Song K, et al. Updated Thresholds of Basal Calcitonin Level and Extent of Lymph Node Metastasis in Initially Treated Medullary Thyroid Cancer. JAMA Otolaryngol Head Neck Surg. 2025 Aug 1;151(8):761-767. doi:10.1001/jamaoto.2025.0542. Erratum in: JAMA Otolaryngol Head Neck Surg. 2025 Nov 13. doi:10.1001/jamaoto.2025.4384. PMID: 40569620; PMCID: PMC12203393.
2. Wells SA Jr, Asa SL, Dralle H, et al. Revised American Thyroid Association Guidelines for the Management of Medullary Thyroid Carcinoma. Thyroid. 2015;25(6):567-610. doi:10.1089/thy.2014.0335.
Author note
This article is a critical synthesis of the referenced multicenter cohort study and current guideline context intended for clinicians and clinical researchers. It is not a substitute for individualized clinical judgment.

This article provides a professional analysis of the utility of updated basal calcitonin thresholds in predicting the extent of lymph node metastasis for patients with medullary thyroid cancer (MTC), and their implications for surgical management.
Study Design:
The research is based on a retrospective, multi-center cohort study involving patients with histologically confirmed MTC who underwent preoperative serum basal calcitonin measurement and subsequent surgical resection. The investigators analyze the association between distinct calcitonin cutoff values and the presence and extent of cervical lymph node metastasis (central/lateral compartments). Receiver operating characteristic (ROC) curves, sensitivity, specificity, and predictive values for different cutoff points are computed. Subgroup analysis evaluates performance in sporadic versus hereditary MTC.
Level of Evidence:
A retrospective multi-center design utilizing real-world surgical and laboratory data offers a robust cohort-level association, though it does not reach the causal strength of prospective trials or randomized studies. Nevertheless, the large sample size, multi-institutional nature, and pathological gold standard outcome ascertainment (postoperative lymph node status) provide strong clinical applicability in the endocrine oncology context.
Key Findings:
Updated, higher basal calcitonin cutoffs (e.g., >250 pg/mL for central node involvement, >550 pg/mL for lateral compartment metastasis) improve preoperative prediction of the extent of lymph node metastasis compared with traditional thresholds.
Sensitivities and specificities for these cutoffs are clinically actionable, and the study proposes practical algorithms for using calcitonin values to stratify patients for surgical planning: e.g., total thyroidectomy with central and lateral neck dissection at higher thresholds.
Subgroup analysis suggests cutoffs remain informative across both sporadic and hereditary MTC, though minor performance differences may exist.
Clinical Implications:
Preoperative stratification using evidence-based calcitonin cutoffs can optimize surgical decision-making in MTC, reducing both the undertreatment and overtreatment of regional disease. This approach supports personalized surgical planning—potentially reducing unnecessary morbidity from extensive lymph node dissection in patients below the cutoffs, while ensuring high-risk patients receive comprehensive nodal clearance. It can also aid in patient counseling and multidisciplinary team discussions.
Ongoing Clinical Issues:
As a retrospective analysis, there are potential biases (e.g., variability in surgical approach, calcitonin assay differences, missing data).
These thresholds may not perfectly capture microscopic nodal disease, and individualized assessment remains necessary, especially in borderline or atypical presentations.
Applicability to diverse populations and across different assay platforms should be further validated.
The influence of other biomarkers (e.g., CEA levels, RET mutation status) in conjunction with calcitonin remains to be fully defined.
Future Research Directions:
Prospective, ideally multi-institutional studies validating these cutoffs and surgical algorithms in newly-diagnosed MTC patients.
Comparative research evaluating combined biomarker panels (e.g., calcitonin, CEA, molecular markers) for even better nodal risk prediction.
Long-term outcomes studies examining how calcitonin-stratified surgical planning affects disease recurrence, survival, and quality of life.
Incorporation of these thresholds into clinical guidelines and assessment of their real-world impact on surgical practice.
In conclusion, this study offers strong evidence that tailored basal calcitonin cutoffs can meaningfully inform surgical strategies for medullary thyroid cancer, promoting more precise and patient-centered care. Further prospective validation and integration into clinical algorithms are warranted to maximize patient outcomes.