Introduction
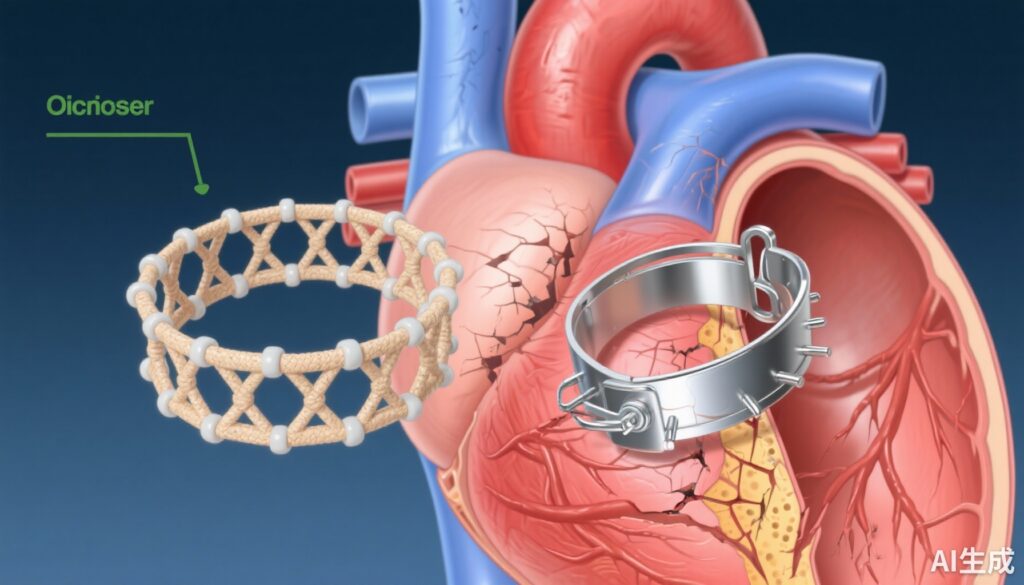
Atrial septal defect (ASD) is a common congenital abnormality characterized by an opening in the septum separating the left and right atria, which can lead to volume overload of the right heart and pulmonary circulation if untreated. Transcatheter closure of ASD has become the standard minimally invasive treatment, predominantly using metallic occluders. While effective, metallic devices pose long-term risks such as erosion, thrombus formation, and interference with subsequent cardiac procedures due to residual hardware. Recent advancements have introduced bioresorbable occluders aimed at overcoming these limitations, but evidence from large, randomized clinical trials remained scarce until now.
Study Background and Rationale
The persistence of complications associated with metallic ASD occluders underscores the need for alternative devices that can provide effective closure without long-term foreign material retention. Bioresorbable occluders represent a promising innovation, potentially reducing late adverse events and facilitating future interventions. This randomized clinical trial by Ouyang et al. aimed to robustly evaluate whether these novel devices are noninferior to traditional metallic plugs in both efficacy and safety, with the additional goal of characterizing their degradation profile.
Study Design and Methods
This multicenter, open-label, noninferiority randomized clinical trial was conducted across 10 hospital sites in China from May 8, 2021, to August 3, 2022. Eligible participants included those with secundum ASD suitable for transcatheter closure. A total of 229 participants completed the procedures, after excluding one participant with inadequate femoral access. Participants were randomized in a 1:1 ratio to receive a bioresorbable occluder (n=116) or a metallic occluder (n=114). The primary endpoint was procedural success in ASD closure at 6 months, evaluated by transthoracic echocardiography, defining closure success as residual shunt diameter ≤2 mm. Secondary endpoints included ASD closure at 2 years, device-related adverse events, and degradation status of the bioresorbable device.
Key Findings
At 6 months, the success rate of ASD closure was 96.5% in the bioresorbable group versus 97.4% in the metallic group, meeting the noninferiority margin (between-group difference of -0.8 percentage points, 95% CI, -5.0 to 3.7; P<.001). The 2-year follow-up reaffirmed these findings, with closure success rates of 94.8% versus 96.5%, respectively (P=.75). Device-related adverse events were rare and comparable — 2.6% in the bioresorbable group and 3.5% in the metallic group (P=.72). Importantly, the bioresorbable occluder demonstrated approximately 99.8% degradation at 2 years, supporting its resorption profile.
The clinical equivalence in closure efficacy and the safety profile suggest that bioresorbable occluders could serve as a viable alternative to metallic devices, with potential benefits including reduced residual foreign material and improved long-term management.
Expert Commentary
This trial marks a significant step forward in transcatheter ASD management. The comparable efficacy and safety profiles indicate that bioresorbable devices can replace metallic occluders without compromising outcomes. The near-complete degradation by two years is promising, as it implies a reduced risk of late complications such as erosion or thrombus formation. However, longer-term follow-up is needed to assess the durability of closure and potential late adverse effects related to device degradation or incomplete resorption.
Limitations include the open-label design and the relatively young median age of participants, which may limit generalizability to adult populations. Additionally, the technical aspects of deploying bioresorbable devices require further standardization and operator training.
Conclusion and Future Directions
The evidence from this randomized trial supports the use of bioresorbable occluders as a noninferior alternative to metallic devices for ASD closure, with the added advantage of biological resorption. This innovation holds promise for enhancing patient safety and reducing long-term device-related complications. Future research should focus on long-term follow-up, optimization of device design, and broader applicability across diverse patient groups.
Funding and Clinical Trial Registration
This study was registered at chictr.org.cn (Identifier: ChiCTR2100044408). Funding sources were not specified but typically involve partnerships between academic and industry investigators.
References
1. Ouyang W, Jiang H, Yan X, et al. Bioresorbable vs Metallic Occluders for Transcatheter Atrial Septal Defect Closure: A Randomized Clinical Trial. JAMA. 2025;10.1001/jama.2025.17639.
2. Du ZD, et al. transcatheter closure of atrial septal defect: current status and future perspectives. Nat Rev Cardiol. 2013;10(8):461-478.
3. Makki FA, et al. Long-term outcomes of atrial septal defect closure devices. Circulation. 2017;135(12):1111-1130.