Introduction
Colorectal cancer (CRC) remains a major global health challenge with high incidence and mortality, particularly among the elderly population. Frailty—a multidimensional clinical syndrome characterized by decreased physiological reserve and increased vulnerability—is notably prevalent in CRC patients, adversely impacting prognosis, treatment tolerance, and survival. As CRC treatments including surgery and chemotherapy impose physiological stress, frailty becomes a critical modifiable risk factor in this population that affects clinical outcomes and quality of life (QoL).
Emerging evidence implicates systemic inflammation as a key mechanistic link between diet and frailty. Diets rich in pro-inflammatory components promote chronic inflammation, which exacerbates muscle wasting and functional decline. Conversely, anti-inflammatory diets that emphasize fruits, vegetables, whole grains, and healthy fats have shown potential in mitigating systemic inflammation and improving physical function.
The dietary inflammatory index (DII) is a validated tool to quantify the overall inflammatory potential of individual diets. Prior studies, including the research by Wang et al., highlight a positive association between higher DII scores and frailty among CRC patients, with inflammatory cytokines such as interleukin-6 (IL-6) and interleukin-10 (IL-10) mediating this relationship. However, interventional data applying DII-guided dietary education to reduce frailty in this clinical setting are limited.
Study Design and Methodology
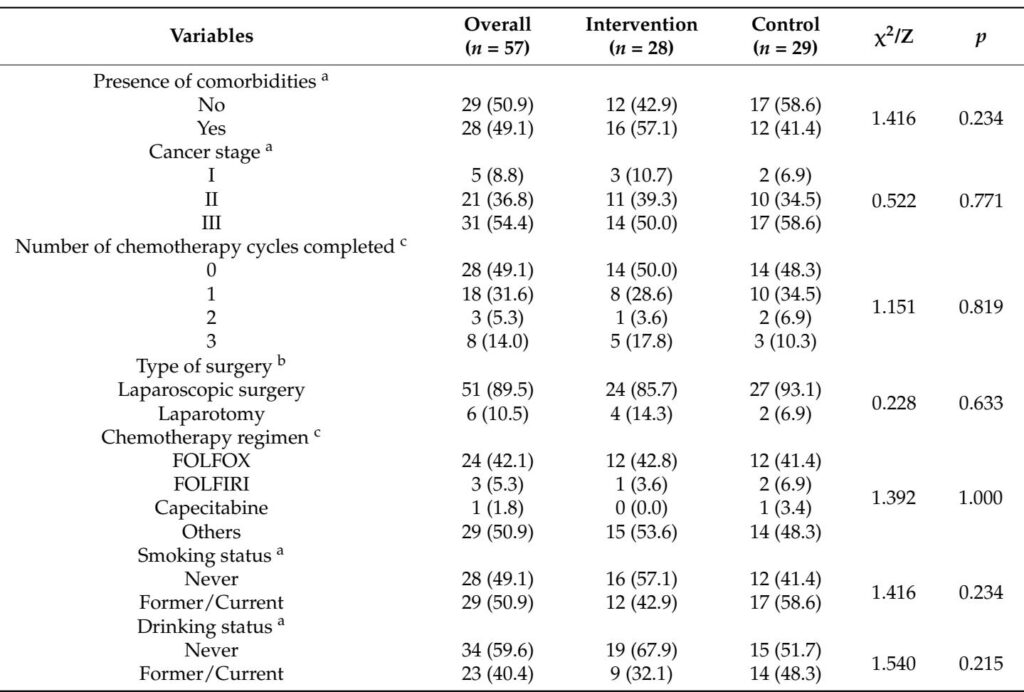
This investigator-initiated, assessor-blinded, randomized controlled trial enrolled 66 frail CRC patients undergoing adjuvant chemotherapy post-curative surgery at Jiangnan University Hospital, China. Participants were adults with stage I-III CRC, meeting Fried frailty phenotype criteria (score ≥ 3). They were randomized 1:1 to receive either a 12-week DII-based anti-inflammatory dietary education program (intervention) or routine health education (control).
The intervention consisted of in-person dietary counseling tailored to reduce overall dietary inflammatory load by increasing intake of anti-inflammatory foods (fruits, vegetables, legumes, nuts, whole grains, healthy fats) and limiting pro-inflammatory components (red/processed meats, fried foods, sugary drinks). Caregivers were also involved to facilitate adherence. Support was continued via WeChat messaging and personalized feedback based on dietary records. The control group received general cancer-related health education without structured dietary guidance.
Primary endpoint was frailty status change measured by the Fried frailty phenotype. Secondary endpoints included changes in DII scores via three-day dietary recalls, plasma IL-6 and IL-10 levels, body mass index (BMI), nutritional status assessed by Mini Nutritional Assessment (MNA), and QoL measured by the Functional Assessment of Cancer Therapy-Colorectal (FACT-C) questionnaire.
Key Findings
Out of 66 randomized patients, 57 (86.4%) completed follow-up. Baseline characteristics were well balanced between groups.
Post-intervention, the intervention group demonstrated statistically significant reductions in DII scores (mean Δ = -0.32, p=0.008) indicating improved dietary anti-inflammatory potential, while controls showed a non-significant increase. Correspondingly, plasma IL-6 decreased and IL-10 increased in the intervention group (p=0.003 and p=0.021, respectively), reflecting systemic inflammation modulation.
Though the between-group difference in frailty prevalence was not statistically significant after 12 weeks, the intervention group exhibited a significant within-group reduction in frailty proportion (p=0.031), while control changes were not significant.
BMI and nutritional status improved significantly in the intervention group (BMI increase, p=0.012; MNA improvement, p=0.027), with stable or declining metrics in controls. Importantly, overall QoL, as measured by FACT-C scores, improved more in the intervention group compared to controls (p=0.014).
Expert Commentary
This study pioneers the application of a DII-based dietary education approach to ameliorate frailty in CRC patients, bridging nutritional epidemiology with practical patient-centered intervention. The observed reduction in dietary inflammatory potential linked to modulation of IL-6 and IL-10 underpins the biological plausibility that diet-induced inflammatory pathways contribute to frailty pathogenesis.
Limitations include the relatively short follow-up period and modest sample size, which may have constrained definitive between-group differences in frailty status. Additionally, the unblinded nature of the intervention to patients and educators is inherent to behavioral trials but addressed by blinded outcome assessment. The chemotherapy context might have offset some intervention benefits due to concurrent treatment-induced systemic stress.
Multi-component interventions integrating dietary modification with physical exercise and psychosocial support may yield synergistic effects and warrant future research. Extended follow-up is essential to evaluate sustained adherence and long-term clinical outcomes.
Conclusion
DII-based anti-inflammatory dietary education significantly improves dietary inflammatory profiles, reduces systemic inflammation, and positively impacts frailty indicators, nutritional status, and quality of life among frail colorectal cancer patients receiving chemotherapy. This low-cost, scalable intervention represents a promising addition to comprehensive CRC supportive care, emphasizing the pivotal role of dietary strategies in mitigating frailty and enhancing survivorship.
References
1. Wang, Y. et al. Effects of 12-Week Dietary Inflammatory Index-Based Dietary Education on Frailty Status in Frail Patients with Colorectal Cancer: A Randomized Controlled Trial. Nutrients 17, 2203 (2025).