Highlights
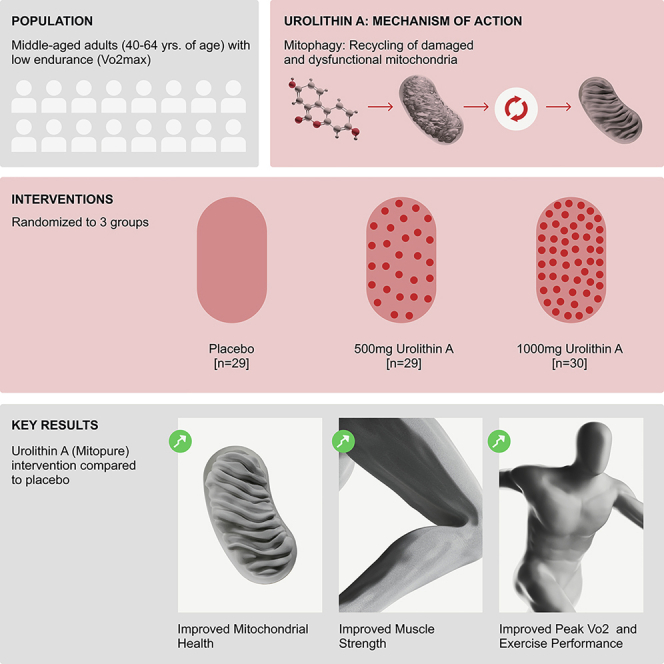
Urolithin A (UA; Mitopure) administered orally for 4 months to middle‑aged, overweight, sedentary adults was safe and associated with: 1) significant improvements in lower‑limb (hamstring) strength (+12% with 500 mg, +9.8% with 1,000 mg versus placebo), 2) clinically meaningful increases in aerobic capacity and walking distance with the 1,000 mg dose (peak VO2 up ~10% within‑group; 6‑minute walk distance +33.4 m), and 3) muscle proteomic and biochemical signatures consistent with activation of Parkin‑mediated mitophagy and elevated mitochondrial OXPHOS proteins, together with reduced systemic CRP and selected inflammatory cytokines.
Study background and clinical need
Age‑related decline in muscle mass, strength and endurance (sarcopenia and dynapenia) is a major driver of disability and loss of independence. Interventions that restore muscle bioenergetics and organelle quality control—specifically mitophagy—are attractive because mitochondrial dysfunction contributes to reduced muscle performance and systemic inflammation with aging. Urolithin A is a gut‑microbiome‑derived metabolite of dietary ellagitannins that has been shown in preclinical studies to activate mitophagy, restore mitochondrial function and improve muscle function (Ryu et al., Nat Med 2016). Early human studies reported safety and molecular signatures of improved mitochondrial health after short‑term UA dosing (Andreux et al., Nat Metab 2019), and a randomized trial in older adults reported improved muscle endurance with longer supplementation (Liu et al., JAMA Netw Open 2022). The ATLAS trial sought to evaluate functional and molecular effects of two UA doses over 4 months in sedentary, overweight middle‑aged adults with low baseline aerobic capacity (VO2max < 35 mL/kg/min).
Study design
This was a single‑center, randomized, double‑blind, placebo‑controlled, parallel‑group proof‑of‑concept trial (NCT03464500). Eighty‑eight participants aged 40–64 years, BMI 25–34.9 kg/m2, sedentary and with low VO2max were randomized to placebo (n=29), UA 500 mg/day (n=29) or UA 1,000 mg/day (n=30) for 120 days. Primary endpoint specified was peak power output (PPO) on a submaximal incremental cycling test. Secondary and exploratory endpoints included isokinetic lower‑limb strength (Biodex dynamometry), hand‑grip strength, peak VO2 (predicted), 6‑minute walk test (6MWT), time to fatigue, DXA body composition, plasma biomarkers (acylcarnitines, CRP, cytokines), muscle transcriptomics and untargeted proteomics, and targeted western blot validation in muscle biopsy subgroups. Analyses were reported for intent‑to‑treat (ITT) and per‑protocol populations; most endpoints were exploratory and the trial was powered as a proof‑of‑concept exercise.
Key findings
Functional outcomes
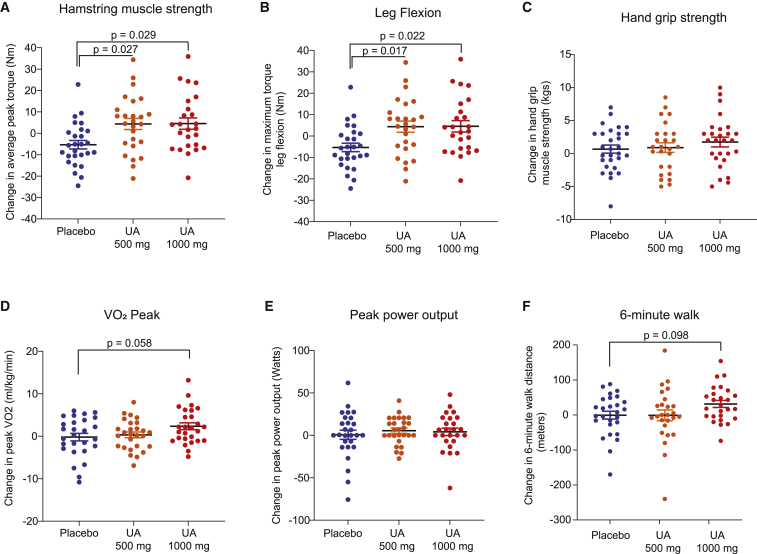
– Lower‑limb strength: Both UA doses produced statistically significant improvements in hamstring strength versus placebo. Average peak torque in hamstrings increased by ~12% (500 mg, p=0.027 vs placebo) and ~9.8% (1,000 mg, p=0.029 vs placebo). Maximum torque during knee flexion improved ~10.5–10.6% with both doses (p≤0.022). By contrast, quadriceps measures trended favorably but did not reach statistical significance. Hand‑grip showed a non‑significant trend (5.1% improvement at 1,000 mg; p=0.08). Lean mass by DXA was unchanged over 4 months.
Aerobic endurance and physical performance
– Primary endpoint: PPO showed non‑significant increases (~4% from baseline in UA groups) and was not different from placebo.
– Peak VO2 and predicted VO2max: The 1,000 mg UA group demonstrated statistically significant within‑group increases in peak VO2 at 2 and 4 months (~10% increase from baseline; p<0.01). When compared with placebo, the between‑group difference approached but did not cross conventional significance thresholds (p≈0.058 for peak VO2).
– 6‑minute walk test: The 1,000 mg UA group increased walking distance by a mean +33.4 m from baseline (p=0.0008 within‑group; p≈0.098 versus placebo). A 30‑m change is commonly considered a minimal clinically important difference in older adults (Perera et al., J Am Geriatr Soc 2006).
– Time to fatigue and cycling distance improved in the 1,000 mg group (cycling distance +15%, p≈0.03 within‑group).
Urolithin A oral administration significantly improves leg muscle strength and impacts aerobic endurance in middle-aged adults
Biomarkers and muscle molecular readouts
– Plasma bioavailability: UA and conjugates (glucuronide, sulfate) were robustly detectable at both doses after 4 months, confirming compliance and systemic exposure.
– Metabolomics: Plasma acylcarnitines (especially medium/long chain species) decreased in the 500 mg group, consistent with improved mitochondrial fatty‑acid oxidation and metabolic efficiency (Schooneman et al., Diabetes 2013). The 1,000 mg group did not show the same acylcarnitine pattern at this timepoint, suggesting possible dose‑ or time‑dependent dynamics.
– Inflammation: High‑sensitivity CRP decreased with UA (statistically significant for 1,000 mg), and selected pro‑inflammatory cytokines (IFN‑γ, IL‑1β, TNF‑α) trended downward collectively, indicating a mild systemic anti‑inflammatory effect.
– Muscle transcriptome and proteome: RNA‑seq revealed enrichment of mitochondrial, ribosomal and contractile fiber gene sets at 500 mg (adjusted p<0.1). Untargeted proteomics demonstrated upregulation of pathways linked to Parkin‑mediated ubiquitin–proteasomal systems (500 mg) and mitochondrial TCA cycle, fatty‑acid oxidation and OXPHOS proteins (1,000 mg). Targeted western blots confirmed increased phospho‑Parkin (Ser65) after 500 mg and dose‑dependent increases in complex I–III OXPHOS proteins, supporting activation of mitophagy and improved mitochondrial protein abundance. A small increase in mtDNA/nDNA ratio was observed.
Safety
UA was well tolerated. Across groups, adverse events were mostly mild and related in part to the muscle‑biopsy procedure; overall AE counts were similar between placebo and UA arms. There were no clinically meaningful changes in vitals, hematology, chemistry panels or urinalysis. No serious safety signals were reported during the 4‑month period.
Mechanistic interpretation and biological plausibility
Preclinical data show UA activates mitophagy via PINK1/Parkin pathways and improves muscle mitochondrial quality and function (Ryu et al., Nat Med 2016). The ATLAS proteomic and western blot data — increased UBE2N, phospho‑Parkin and OXPHOS proteins — are congruent with mitophagy activation leading to rejuvenated mitochondrial networks and higher oxidative capacity. The observed reductions in acylcarnitines and CRP are physiologically consistent with improved mitochondrial fatty‑acid handling and reduced inflamm‑aging, both of which can contribute to improved muscle endurance and walking performance.
Limitations and expert perspective
This remains a proof‑of‑concept single‑center study with exploratory endpoints. The pre‑specified primary endpoint (PPO) was not met, and several important outcomes showed within‑group rather than robust between‑group significance. Sample size and single‑site recruitment limit generalizability. The population was overweight, sedentary, and predominantly female (~2:1), which may not represent frail older adults with sarcopenia. Some molecular signals differed by dose and modality (transcriptomics vs proteomics), reflecting the complexity of time‑ and dose‑dependent pharmacodynamics. Additionally, the trial was sponsored by the product developer; independent replication is desirable. Despite these caveats, the convergence of functional improvements (notably hamstring strength and clinically meaningful 6MWT gain at 1,000 mg), systemic biomarker changes, and proteomic evidence of mitophagy activation provide a plausible, mechanistically coherent signal that warrants confirmatory trials.
Clinical implications and recommended next steps
For clinicians, UA is not yet an approved therapy for sarcopenia, but these data suggest UA merits further study as a nutritional or adjunctive strategy to preserve or improve muscle strength and endurance, particularly when exercise is limited or adherence is poor. Future trials should be adequately powered, multicenter, of longer duration (≥6–12 months), include older adults and patients with sarcopenia, pre‑specify primary endpoints that were positive here (e.g., hamstring torque or 6MWT or peak VO2), examine dose–response and time course, and include objective activity monitoring. Mechanistic substudies should continue with standardized biopsy timing to reconcile transcriptomic and proteomic changes, and explore effects on clinical outcomes (falls, mobility disability) and metabolic endpoints (insulin sensitivity).
Conclusion
The ATLAS randomized trial demonstrates that four months of oral urolithin A is safe, bioavailable, and associated with improved lower‑limb strength, signals of improved aerobic capacity and walking ability (particularly at 1,000 mg), reduced inflammatory biomarkers, and muscle proteomic signatures consistent with activation of Parkin‑mediated mitophagy and enhanced mitochondrial protein content. These findings align with preclinical evidence and earlier human studies, and support larger, confirmatory trials to determine whether UA can be an effective intervention to preserve muscle function with aging.
Key references
1. Ryu D, et al. Urolithin A induces mitophagy and prolongs lifespan in C. elegans and increases muscle function in rodents. Nat Med. 2016;22(8):879–888.
2. Andreux PA, et al. The mitophagy activator urolithin A is safe and induces a molecular signature of improved mitochondrial and cellular health in humans. Nat Metab. 2019;1:595–603.
3. Liu S, et al. Effect of urolithin A supplementation on muscle endurance and mitochondrial health in older adults: a randomized clinical trial. JAMA Netw Open. 2022;5(1):e2144279.
4. Perera S, et al. Meaningful change and responsiveness in common physical performance measures in older adults. J Am Geriatr Soc. 2006;54:743–749.
5. Schooneman MG, et al. Acylcarnitines. Diabetes. 2013;62(1):1–8.