Highlight
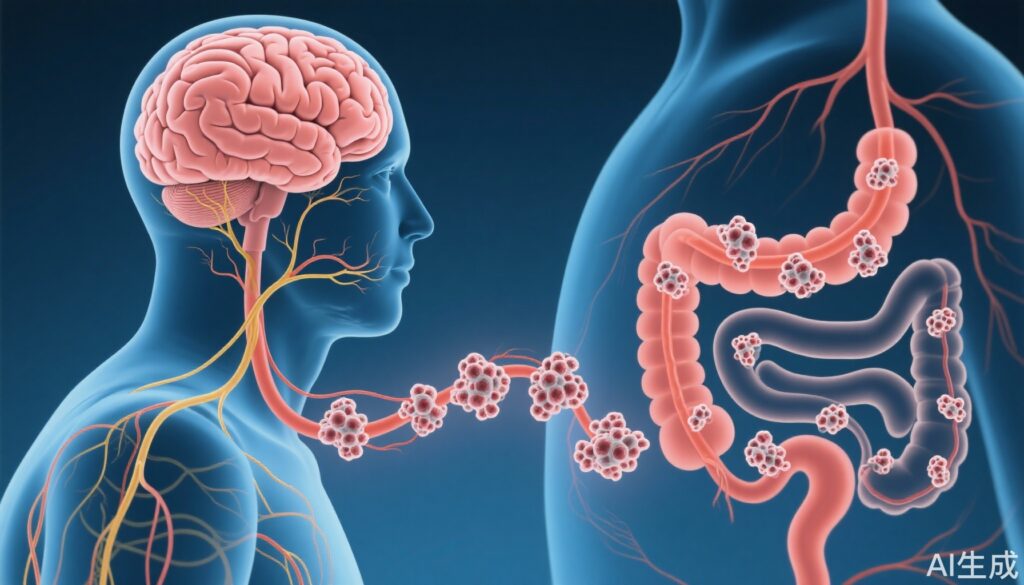
- Pathological tau spreads from the hippocampus through the vagal efferent neurons to the enteric nervous system in the colon in Alzheimer’s disease (AD) models.
- Vagotomy markedly reduces tau transmission to the colon, indicating a critical role of the vagus nerve in tau propagation.
- An innovative innervated colon-on-a-chip model replicates the gut–brain axis, demonstrating tau transmission impact on colon epithelial stability.
- Findings provide direct evidence of brain-to-gut pathological tau propagation, underscoring the importance of the vagus nerve in AD pathology beyond the central nervous system.
Study Background and Disease Burden
Alzheimer’s disease (AD) is a progressive neurodegenerative disorder characterized clinically by cognitive decline and neuropathologically by amyloid-β (Aβ) plaque deposition and tau neurofibrillary tangles in the brain. It represents a profound public health challenge with immense socio-economic burdens worldwide due to its increasing prevalence amongst aging populations.
Beyond central nervous system (CNS) pathology, emerging evidence suggests that AD may influence peripheral organs, particularly via the gut–brain axis (GBA). The GBA is a bidirectional communication system involving neuronal, immune, and endocrine crosstalk between the CNS and the gastrointestinal tract. Prior studies identified alterations in gut microbiota and intestinal dysfunction in AD but lacked mechanistic insights into how AD pathology might directly affect gut tissues.
Understanding how tau pathology might propagate beyond the brain is crucial for revealing novel aspects of AD pathogenesis. This could open new therapeutic avenues targeting gut involvement in AD and enhance our understanding of systemic disease manifestations.
Study Design
The study by Choi et al. employed a multifaceted experimental approach. First, they used the ADLPAPT transgenic mouse model, which expresses human tau mutations to recapitulate AD tau pathology. The aim was to trace the propagation of pathological tau from the brain to the colon.
Key components of the study design included:
– Immunohistochemical and biochemical analysis for tau aggregates in the hippocampus, dorsal motor nucleus of the vagus (DMV), and colon nerve plexuses.
– Surgical vagotomy (vagal nerve cut) in ADLPAPT mice to determine if the vagus nerve mediates tau spread.
– Construction of an innovative innervated colon-on-a-chip model integrating human induced pluripotent stem cell (iPSC)-derived vagal motor neurons, enteric neurons, and colon epithelial cells to simulate the gut–brain axis in vitro.
Endpoints assessed were tau propagation along the vagus nerve pathways, presence and distribution of tau aggregates in colon tissue, and effects on colon epithelium structural integrity.
Key Findings
In the ADLPAPT mouse model, tau pathology was detectable not only in the brain structures characteristic for AD but also in the nerve plexuses of the colon—suggesting peripheral nervous system involvement.
Specifically:
– Tau aggregates were consistently observed in the hippocampus, and notably, tau spread to the dorsal motor nucleus of the vagus, a brainstem region responsible for vagal efferent output.
– Tau pathology extended further from the DMV to the enteric neurons within the colon’s nerve plexuses.
– Vagotomized ADLPAPT mice exhibited a marked reduction in tau presence in the colon, supporting the neural pathway hypothesis that the vagus nerve mediates tau transmission from brain to gut.
The colon-on-a-chip system recapitulated this propagation pathway in vitro:
– Vagal motor neurons transmitting tau aggregates successfully transmitted tau pathology to downstream enteric neurons.
– This tau transmission was associated with impaired colon epithelial barrier integrity, suggesting a functional consequence on gut physiology.
Together, the findings demonstrate a mechanistic pathway where pathological tau moves via the vagal efferent fibers, affecting enteric neurons and compromising colon epithelial stability.
Expert Commentary
These findings address a critical gap in AD research by demonstrating direct pathological tau propagation from the brain to peripheral organs via a defined anatomical pathway. The vagus nerve’s involvement fits well with known neuroanatomical connectivity and complements emerging data on the gut–brain axis role in neurodegenerative diseases.
Limitations include reliance on a single transgenic mouse model and in vitro simulations, which may not capture all complexities of human physiology. Additionally, while the study demonstrates impact on colon epithelium, the direct clinical relevance relating to gastrointestinal symptoms in AD patients requires further validation.
Experts acknowledge the study’s elegant combination of in vivo and organ-on-chip techniques as a powerful platform to dissect complex neuroimmune interactions beyond the CNS. Future research may investigate whether targeting vagal pathways or enteric tau aggregates could mitigate AD progression.
Conclusion
Choi et al.’s study offers robust evidence that pathological tau can propagate from the brain to the colon through the vagal efferent pathway in Alzheimer’s disease. This paradigm-shifting discovery extends the pathological spectrum of AD beyond the CNS, implicating peripheral nervous system involvement via the gut–brain axis.
These insights deepen our understanding of AD pathophysiology and suggest that interventions aimed at the vagus nerve or peripheral tau deposits represent potential novel therapeutic strategies. Moreover, the use of innervated colon-on-a-chip models provides a versatile tool for future mechanistic and pharmacological studies focusing on gut involvement in neurodegeneration.
Ongoing and future studies should explore the impact of this transmission on gastrointestinal symptoms, systemic inflammation, and AD progression in humans to translate these findings clinically.
References
Choi H, Hong SB, Liu HW, Song H, Jeong JH, Ahn K, Kim S, Song J, Han JW, Lee D, Ahn J, Kim MS, Chung S, Mook-Jung I. Pathological tau propagation from the brain to the colon via the vagal efferent pathway in Alzheimer’s disease. Gut. 2025 Sep 1:gutjnl-2024-334571. doi: 10.1136/gutjnl-2024-334571. Epub ahead of print. PMID: 40890024.
Additional relevant literature:
– Holmqvist et al., 2014. Direct evidence of Parkinson pathology spread from the gastrointestinal tract to the brain in rats. Acta Neuropathol.
– Pan-Montojo et al., 2010. Environmental toxins trigger PD-like progression via the gut. Neurobiol Dis.
– Kim et al., 2020. The gut microbiota and Alzheimer’s disease. J Alzheimers Dis.
– Wang and Wang, 2022. Role of the gut–brain axis in neurodegenerative diseases. Front Neurosci.