Background
Tumor immunotherapy has emerged as a pivotal advancement in cancer treatment, harnessing immune system mechanisms to target malignant cells with improved specificity. Nevertheless, the tumor microenvironment (TME) — characterized by heterogeneous physiological conditions including acidity, elevated reductive potential, and abnormal energy metabolism — poses significant barriers to effective immune surveillance and therapy. Key challenges include immune evasion mediated by TME-induced immunosuppression and altered metabolic states like the Warburg effect in tumor cells, which reduce treatment responsiveness.
Moreover, the cyclic GMP-AMP synthase (cGAS)-stimulator of interferon genes (STING) pathway is recognized as an essential innate immune mechanism capable of initiating antitumor immunity through type I interferon and pro-inflammatory cytokine production. While manganese ions (Mn2+) have recently been identified as potent activators of the cGAS-STING signaling cascade and facilitators of immune cell activation, their clinical efficacy is hampered by nonspecific distribution and potential systemic toxicity.
Thus, there exists a critical need for strategies enabling targeted delivery of Mn2+, combined with the modulation of tumor metabolism and the TME to potentiate immunotherapy.
Study Design
This study introduces a novel nanoplatform, LT@MnO@MON-HA (LMMH), engineered for tumor microenvironment responsiveness enabling controlled release of manganese ions (Mn2+) and the mitochondrial glycolysis inhibitor lonidamine (LT). The methodology involves:
– Synthesis of manganese oxide (MnO) nanoparticles encapsulated within an organic mesoporous silica (MON) shell containing disulfide bonds enabling glutathione (GSH)-responsive degradation.
– Encapsulation of mitochondria-targeted lonidamine (LT) within mesopores, followed by surface coating with hyaluronic acid (HA) for tumor-specific targeting via CD44 receptor affinity.
– Comprehensive physicochemical characterization including transmission electron microscopy, dynamic light scattering, zeta potential measurements, and in vitro assays assessing Fenton-like catalytic activity and drug release in TME-mimicking conditions.
– Evaluation of intracellular ROS generation, metabolic interference on glycolysis and pentose phosphate pathways in 4T1 tumor cells.
– Activation of the cGAS-STING pathway assessed in bone marrow-derived dendritic cells via Western blotting, qRT-PCR, and cytokine ELISAs.
– In vivo efficacy exploration through a bilateral 4T1 tumor-bearing mouse model with detailed tumor growth assessment, immune cell infiltration, cytokine profiling, and toxicity analysis.
Statistical analyses involved ANOVA and t-test comparisons, ensuring rigor in data interpretation.
Key Findings
Nanoplatform Characterization and Tumor Microenvironment Responsiveness
The synthesized LMMH nanoplatform exhibited a distinct core-shell structure with MnO nanoparticles at its core shielded by a mesoporous silica shell incorporating GSH-responsive disulfide bonds. TEM and elemental mapping confirmed manganese encapsulation within silica matrices. LMMH demonstrated colloidal stability over one week under physiological conditions and showed acid- and GSH-triggered release of Mn2+ ions and LT, with HA coating providing sustained release and enhanced tumor-targeting capability. In vitro, LMMH catalyzed endogenous H2O2 conversion to highly reactive hydroxyl radicals (·OH) via Fenton-like reactions, enabling effective chemodynamic therapy (CDT). Notably, LMMH consumed GSH efficiently in reductive conditions, further facilitating oxidative stress in tumor cells.
Metabolic Inhibition and Enhanced ROS Generation
LT incorporated within LMMH targeted mitochondria to disrupt tumor glycolysis and the pentose phosphate pathway, as evidenced by reduced glucose-6-phosphate and lactate levels, and increased NADP+/NADPH ratio in treated 4T1 cells. This metabolic interference amplified intracellular ROS levels and synergized with Mn2+-catalyzed ·OH generation, resulting in heightened tumor cell apoptosis through oxidative stress mechanisms. These effects were superior to free LT or MnO formulations alone, highlighting enhanced drug delivery and biological activity of the nanoformulation.
Robust Activation of cGAS-STING Pathway and Immunogenic Cell Death (ICD)
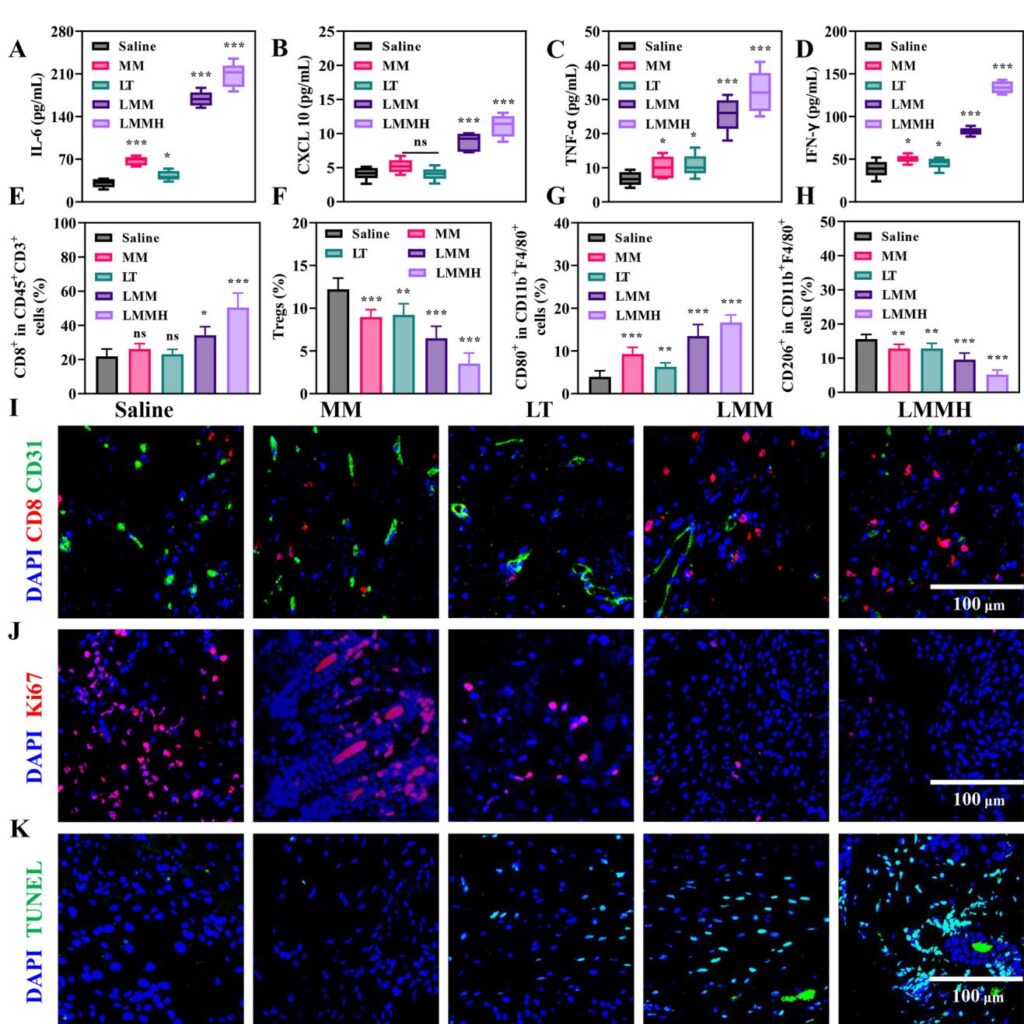
LMMH effectively activated the cGAS-STING pathway in dendritic cells, demonstrated by increased phosphorylation of STING and IRF3, and elevated expression and secretion of type I interferons (IFN-β), pro-inflammatory cytokines (IL-6, TNF-α), and chemokines (CXCL10, CCL5). This immune activation induced potent immunogenic cell death signatures in tumor cells, including enhanced calreticulin surface exposure, HMGB1 release, and ATP secretion, which promote antigen presentation and stimulate T cell-mediated adaptive immunity.
In Vivo Therapeutic Efficacy and Immune Modulation
In a bilateral murine 4T1 breast cancer model, LMMH profoundly inhibited primary and distant tumor growth with a tumor suppression rate exceeding 90%, outperforming free LT, MnO@MON (MM), or their uncombined counterparts. LMMH-treated tumors showed increased infiltration of cytotoxic CD8+ T cells, elevated M1 macrophage polarization, and reduced regulatory T cells and M2-like macrophages, collectively remodeling the immunosuppressive TME. Correspondingly, intratumoral levels of antitumor cytokines IL-6, CXCL10, TNF-α, and IFN-γ were significantly elevated. Additionally, immunofluorescence revealed focal suppression of angiogenesis and proliferative markers with enhanced tumor cell apoptosis. Importantly, the nanoplatform exhibited a favorable safety profile with no significant systemic toxicity or organ damage observed.
Expert Commentary
The LMMH nanoplatform represents an elegant integration of nanotechnology, immunology, and tumor metabolism modulation. By combining mitochondrial metabolic interference with targeted Mn2+-mediated cGAS-STING activation, this strategy overcomes critical hurdles imposed by the hostile TME. The GSH-responsive silica shell appears advantageous in tuning release kinetics and amplifying oxidative stress. This dual therapeutic mechanism—metabolic disruption and innate immune stimulation—can synergize to break tumor immune evasion and potentiate systemic antitumor immunity, including distant metastasis control.
Nonetheless, translation to clinical use requires addressing the complexity and scalability of nanomaterial synthesis, pharmacokinetics, and long-term safety. The biodistribution and potential off-target effects of Mn-based agents warrant further elaboration. Moreover, combining LMMH with checkpoint inhibitors or other immune modulators could be explored to further augment efficacy. Future studies should consider diverse tumor models and immune microenvironments to corroborate generalizability. Overall, this approach holds promise in advancing immune-metabolic combinatorial cancer therapies.
Conclusion
The tumor microenvironment-responsive Mn-based nanoplatform LT@MnO@MON-HA (LMMH) effectively interfaces metabolic inhibition via mitochondrial targeting and innate immune activation through cGAS-STING pathway stimulation. This dual-mechanism strategy facilitates chemodynamic therapy and immunotherapy synergism to robustly suppress tumor growth and metastasis in preclinical models. The design leverages TME-specific triggers like acidity and reductive potential for precise cargo release, minimizing systemic toxicity. Metabolic disruption enhances oxidative stress, sensitizing tumor cells to immune-mediated killing, while Mn2+ ions promote cytokine production and immune cell activation. This advanced nanoplatform provides a promising avenue for next-generation combination immunotherapies, addressing critical barriers posed by tumor heterogeneity and immune evasion. Clinical translation efforts should focus on optimizing synthesis protocols and validating safety in diverse tumor contexts. Altogether, LMMH exemplifies the therapeutic potential of integrating tumor metabolic modulation with innate immune pathway engagement to improve cancer patient outcomes.
Reference
Wen E, Tian Y, Chen Y, Wang Z, Feng Y, Liao Z. Tumor microenvironment responsive Mn-based nanoplatform activate cGAS-STING pathway combined with metabolic interference for enhanced anti-tumor therapy. J Nanobiotechnology. 2025 May 25;23(1):377. doi: 10.1186/s12951-025-03453-4IF: 12.6 Q1 . PMID: 40414874IF: 12.6 Q1 ; PMCID: PMC12105397IF: 12.6 Q1 .