Colorectal cancer liver metastases (CRLM) present a significant clinical challenge, with approximately 80% of patients initially diagnosed as having unresectable disease due to extensive tumor burden or critical lesion locations. Systemic induction chemotherapy aims to downsize tumors, enabling potential secondary local interventions, or to prolong survival in palliative contexts. Currently, tumor response assessment largely relies on the RECIST1.1 criteria, which measure changes in the longest diameter of up to two target lesions per organ. However, RECIST1.1 has notable shortcomings, including limited reproducibility and insufficient representation of overall tumor burden, as it neglects volumetric changes across all metastases. Advances in imaging and deep learning have made volumetric assessment of total tumor volume (TTV) feasible, with preliminary data suggesting prognostic superiority to RECIST1.1. Yet, the clinical validity, generalizability, and predictive utility of TTV in patients undergoing systemic induction therapy remain to be elucidated.
Study Design and Methods
This ancillary study analyzed data from the phase 3 CAIRO5 trial (NCT02162563), including 425 patients with initially unresectable liver-only CRLM who received induction systemic chemotherapy. Patients were randomized based on primary tumor location and RAS/BRAF mutation status to receive systemic regimens including FOLFOX-/FOLFIRI-bevacizumab, FOLFOXIRI-bevacizumab, or FOLFOX-/FOLFIRI-panitumumab. Contrast-enhanced CT scans prior to treatment and at first follow-up were used to quantify baseline TTV and changes post-treatment using a semi-automatic volumetric segmentation approach validated by expert radiologists. RECIST1.1 assessments, including sum of diameters and categorization of response, were performed by an expert panel. The main endpoint was overall survival (OS), with statistical analyses employing multivariable Cox regression models adjusted for baseline clinical and molecular variables, and flexible parametric survival modeling to assess prognostic and predictive capacities of TTV relative to RECIST1.1.
Key Findings
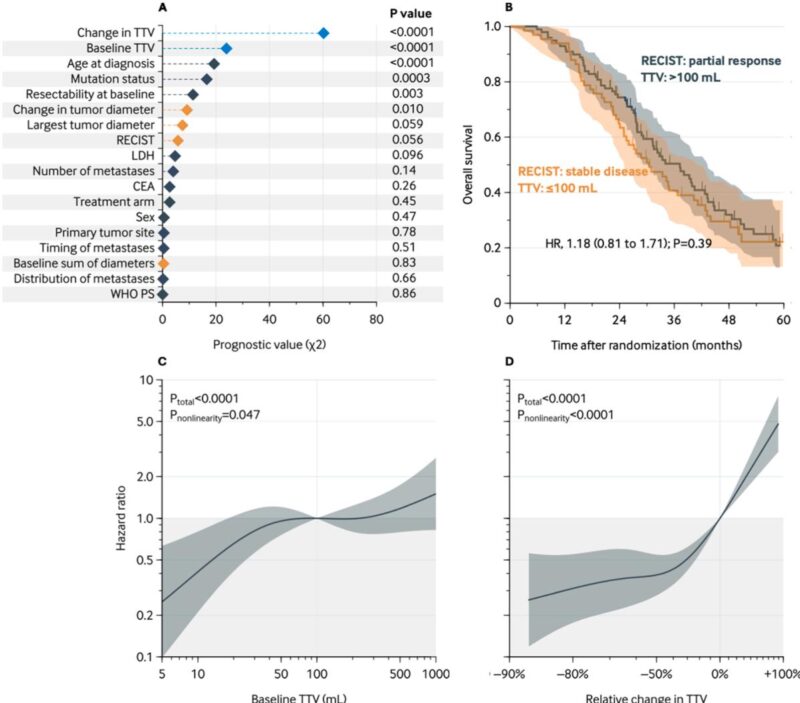
Baseline median TTV was 103 mL (IQR, 25–409 mL); median relative tumor volume reduction post-treatment was 51%. Importantly, TTV and its relative change were the strongest independent predictors of OS (adjusted hazard ratio [aHR] for 100 mL vs 10 mL baseline TTV: 2.44; 95% CI, 1.25–4.76; P=0.006; aHR for 0% vs 50% decrease in TTV: 2.57; 95% CI, 1.83–3.60; P<0.0001). In contrast, RECIST1.1 response categories showed no independent association with OS (aHR partial response vs progressive disease: 0.63; 95% CI, 0.33–1.20). Patients with partial response but high baseline TTV had OS comparable to those with stable disease and low baseline TTV, underscoring the superior prognostic discrimination offered by TTV.
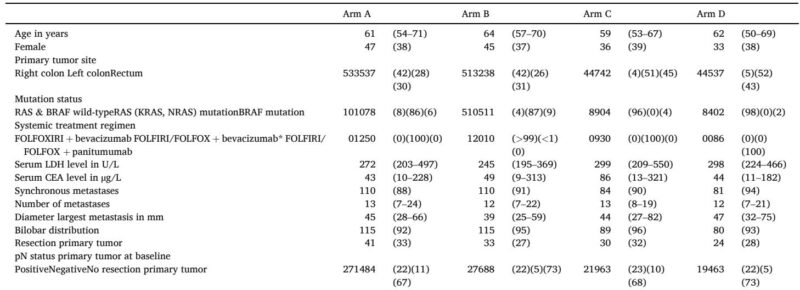
Further, a nonlinear relationship between TTV measures and survival was observed. A baseline TTV of 100 mL was associated with markedly worse OS compared to 5 mL, though risk plateaued beyond 100 mL. Similarly, survival benefits from TTV reductions were significant up to approximately 50% reduction, with diminishing returns thereafter. Notably, OS prediction improved by incorporating both baseline TTV and change in TTV alongside clinical variables, elevating the C-statistic from 0.71 to 0.75 (P=0.002).
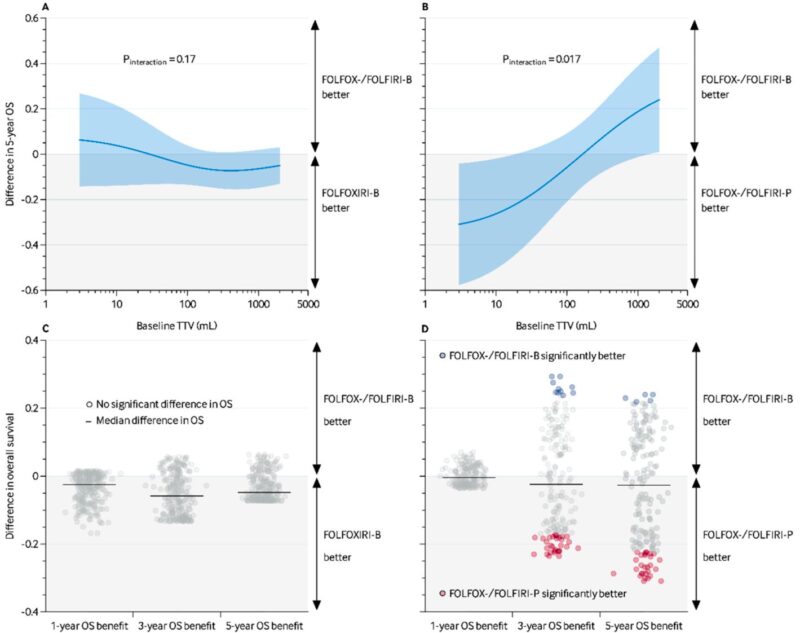
Predictive analyses revealed that higher baseline TTV predicted greater OS benefit from FOLFOX-/FOLFIRI-bevacizumab versus panitumumab in patients with left-sided, RAS/BRAF wild-type tumors (P interaction=0.017). For instance, at 3 mL baseline TTV, panitumumab yielded superior 5-year survival (60% vs 29%), whereas at 2000 mL, bevacizumab was favored (24% survival benefit). No predictive value of TTV was observed in patients with right-sided or mutated tumors regarding bevacizumab regimens.
Expert Commentary
This robust analysis leveraging phase 3 trial data and advanced imaging quantification substantiates TTV as a superior biomarker over RECIST1.1 for both prognostication and early treatment response evaluation in unresectable CRLM. TTV’s volumetric nature addresses the limitations of diameter-based RECIST assessments, capturing the full tumor burden and biologically relevant changes in tumor mass. Incorporating both baseline burden and volumetric changes provides nuanced stratification critical for personalized management decisions.
The potential of TTV to guide therapeutic choices—especially in selecting between bevacizumab and panitumumab in RAS/BRAF wild-type left-sided primaries—is particularly intriguing but requires mechanistic exploration. These findings may shift clinical paradigms from purely anatomical assessments of resectability towards biologically informed treatment strategies. Importantly, the manual segmentation requirement currently limits widespread adoption; ongoing automation efforts should enhance clinical feasibility.
Study limitations include the exclusive focus on initially unresectable liver-only disease, necessitating validation in resectable and multi-organ metastatic contexts. The early closure of certain trial arms and limited molecular subtyping preclude definitive conclusions on regimen-specific predictive effects. Furthermore, clinical implementation requires tailored cut-offs for TTV reduction, as uniform thresholds may not capture heterogeneity in baseline tumor burden.
Conclusion
Total tumor volume and its early change post-chemotherapy offer compelling prognostic and predictive value beyond RECIST1.1 in patients with initially unresectable colorectal liver metastases. Integration of TTV assessment can refine survival prediction, enable more sensitive response monitoring, and potentially personalize the selection of targeted systemic therapies such as bevacizumab or panitumumab. Pending further validation and technological enhancements for automated volumetric analysis, TTV represents a promising imaging biomarker poised to enhance multidisciplinary management in advanced colorectal cancer.
References
1. Adam R et al. The oncosurgery approach to managing liver metastases from colorectal cancer: a multidisciplinary international consensus. Oncologist. 2012;17(10):1225–39.
2. de Ridder JAM et al. Management of liver metastases in colorectal cancer patients: a retrospective case-control study of systemic therapy versus liver resection. Eur J Cancer. 2016;59:13–21.
3. Angelsen JH et al. Population-based study on resection rates and survival in patients with colorectal liver metastasis in Norway. Br J Surg. 2017;104(5):580–9.
4. Van den Eynde M, Hendlisz A. Treatment of colorectal liver metastases: a review. Rev Recent Clin Trials. 2009;4(1):56–62.
5. Kopetz S et al. Improved survival in metastatic colorectal cancer is associated with adoption of hepatic resection and improved chemotherapy. J Clin Oncol. 2009;27(22):3677–83.
6. Poston GJ et al. Urgent need for a new staging system in advanced colorectal cancer. J Clin Oncol. 2008;26(29):4828–33.
7. Adam R et al. Rescue surgery for unresectable colorectal liver metastases downstaged by chemotherapy: a model to predict long-term survival. Ann Surg. 2004;240(4):644–57.
8. Adam R, Kitano Y. Multidisciplinary approach of liver metastases from colorectal cancer. Ann Gastroenterol Surg. 2019;3(1):50–6.
9. Lam VW et al. A systematic review of clinical response and survival outcomes of downsizing systemic chemotherapy and rescue liver surgery in patients with initially unresectable colorectal liver metastases. Ann Surg Oncol. 2012;19(4):1292–301.
10. Adam R. Developing strategies for liver metastases from colorectal cancer. Semin Oncol. 2007;34(2 1):S7–11.
11. Rothe JH et al. Size determination and response assessment of liver metastases with computed tomography–comparison of RECIST and volumetric algorithms. Eur J Radiol. 2013;82(11):1831–9.
12. Laubender RP et al. Evaluating the agreement between tumour volumetry and the estimated volumes of tumour lesions using an algorithm. Eur Radiol. 2014;24(7):1521–8.
13. Eisenhauer EA et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–47.
14. Bereska JI et al. Development and external evaluation of a self-learning auto-segmentation model for colorectal cancer liver metastases assessment (COALA). Insights Imaging. 2024;15(1):279.
15. Michiel Zeeuw J et al. Prognostic value of total tumor volume in patients with colorectal liver metastases: a secondary analysis of the randomized CAIRO5 trial with external cohort validation. Eur J Cancer. 2024;207:114185.
16. Bond MJG et al. First-line systemic treatment strategies in patients with initially unresectable colorectal cancer liver metastases (CAIRO5): an open-label, multicentre, randomised, controlled, phase 3 study. Lancet Oncol. 2023;24(7):757–71.
