Highlights
- Sequential administration of pembrolizumab after chemoradiation significantly improved 4-year locoregional control (LRC) compared to concurrent administration in locally advanced head and neck cancer.
- Both treatment strategies met efficacy and safety benchmarks, but sequential therapy showed numerically better progression-free and overall survival.
- Distinct immunosuppressive changes in the tumor microenvironment were observed based on the timing of pembrolizumab, potentially informing future immunotherapy strategies.
- This randomized Phase II trial addresses a critical question regarding optimal integration of immunotherapy with standard chemoradiation in high-risk HNSCC.
Study Background and Disease Burden
Head and neck squamous cell carcinoma (HNSCC) represents a group of aggressive malignancies with substantial morbidity and mortality, particularly in locally advanced stages. Despite standard concurrent chemoradiation (CRT) with cisplatin, outcomes remain suboptimal, with significant risk of locoregional and distant failure. The advent of immune checkpoint inhibitors, such as pembrolizumab, has transformed the therapeutic landscape in recurrent/metastatic settings. However, the optimal timing of pembrolizumab in combination with CRT for newly diagnosed, locally advanced disease remains unclear. This question is of high clinical relevance, as integrating immunotherapy could enhance tumor control but also risks overlapping toxicities and potentially immunosuppressive interactions with radiation or chemotherapy.
Study Design
This was a randomized, open-label, Phase II trial (NCT02777385) enrolling 80 patients with newly diagnosed, locally advanced HNSCC (predominantly stage IV, T4 and/or N2 disease). Eligibility included both HPV-positive (with additional risk factors) and HPV-negative tumors. Patients were stratified by HPV and N stage and randomized 1:1 to:
- Concurrent Arm: Pembrolizumab 200 mg every 3 weeks for 8 cycles, starting 1 week before CRT (cisplatin 40 mg/m2 weekly + radiation 70 Gy)
- Sequential Arm: Pembrolizumab 200 mg every 3 weeks for 8 cycles, starting 2 weeks after completion of CRT
The primary composite endpoint required each arm to meet all three criteria: 1-year locoregional failure (LRF) rate <60%, 1-year progression-free survival (PFS) ≥60%, and dose-limiting toxicity ≤20%. If both arms met these, the arm with higher 1-year PFS would be considered superior (pick-the-winner design). Secondary endpoints included 4-year locoregional control (LRC), PFS, overall survival (OS), and translational analyses of tumor microenvironment (TME) changes.
Key Findings
Patient Population: Of the 80 patients treated (41 concurrent, 39 sequential), 92.5% had stage IV disease, with a high incidence of bulky and/or node-positive tumors.
Efficacy Outcomes:
- Both arms achieved prespecified primary efficacy and safety endpoints: 1-year LRF (concurrent 26%, sequential 13%), 1-year PFS (concurrent 60%, sequential 74%), dose-limiting toxicity (both ≤20%).
- 4-year Locoregional Control (LRC): Sequential arm had markedly superior LRC (96% vs 64%; HR 0.11, 95% CI 0.01–0.89, P=0.012).
- 4-year Progression-Free Survival (PFS): Sequential arm showed numerically higher PFS (69% vs 49%; HR 0.55, 95% CI 0.25–1.22, P=0.132), not statistically significant.
- 4-year Overall Survival (OS): Sequential arm numerically outperformed concurrent arm (83% vs 71%; HR 0.51, 95% CI 0.19–1.37, P=0.17).
- In patients receiving optimal cisplatin dose (≥200 mg/m2), the sequential arm still demonstrated superior 4-year LRC (96% vs 73%; HR 0.17, 95% CI 0.02–1.43, P=0.063).
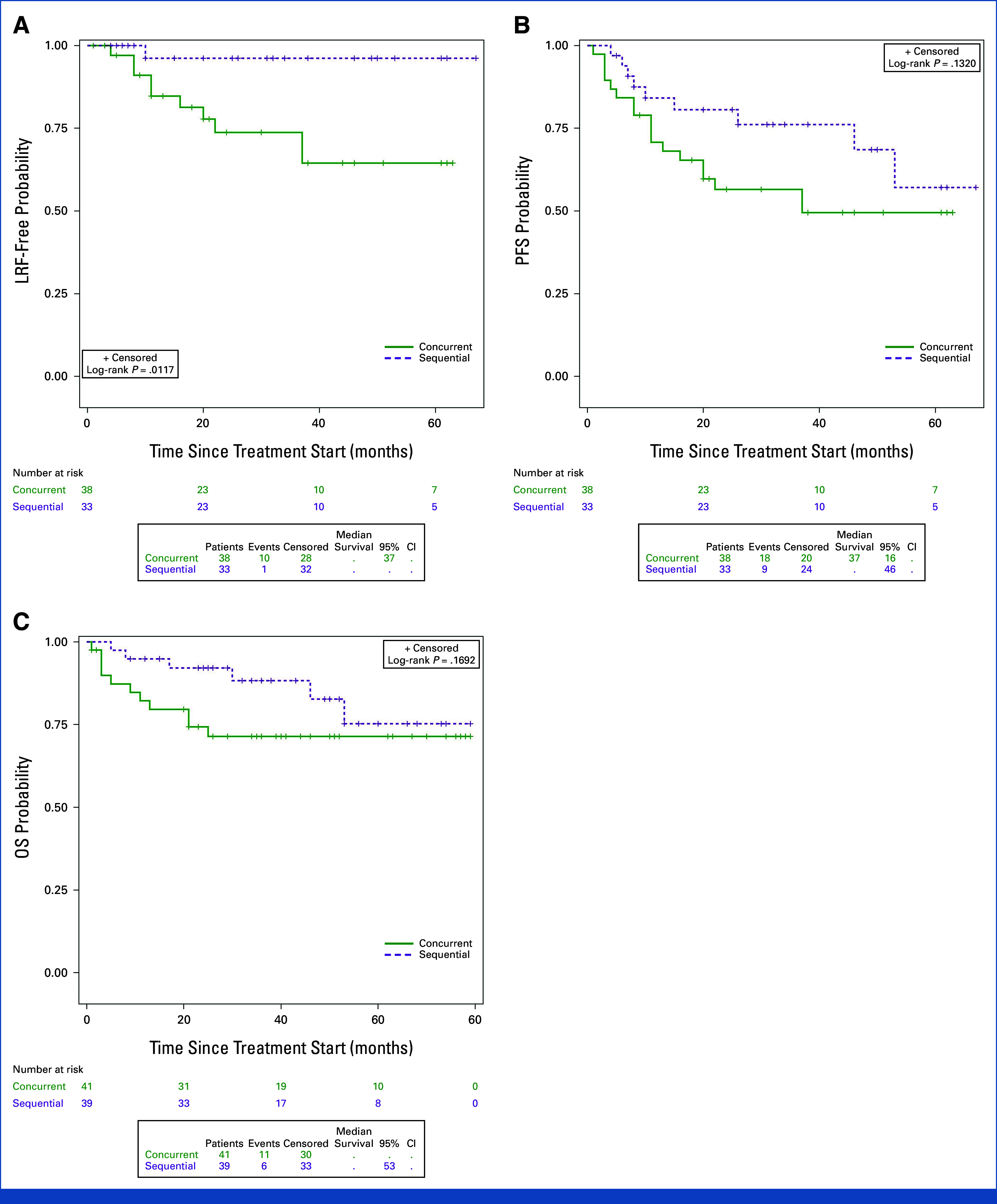
Evaluation of outcomes by treatment arm.
Patterns of Failure: Most progressions in the sequential arm were distant (5/6), while the concurrent arm exhibited more locoregional failures (8/12).
Safety: Both strategies had manageable toxicity profiles, with no unexpected safety signals or significant difference in compliance.
Translational Insights: Tumor biopsies revealed a significant increase in macrophages, PD-L1+ macrophages, and PD-L1+ tumor cells with concurrent but not sequential pembrolizumab, indicating early immunosuppressive changes in the TME potentially driven by initial pembrolizumab exposure before CRT.
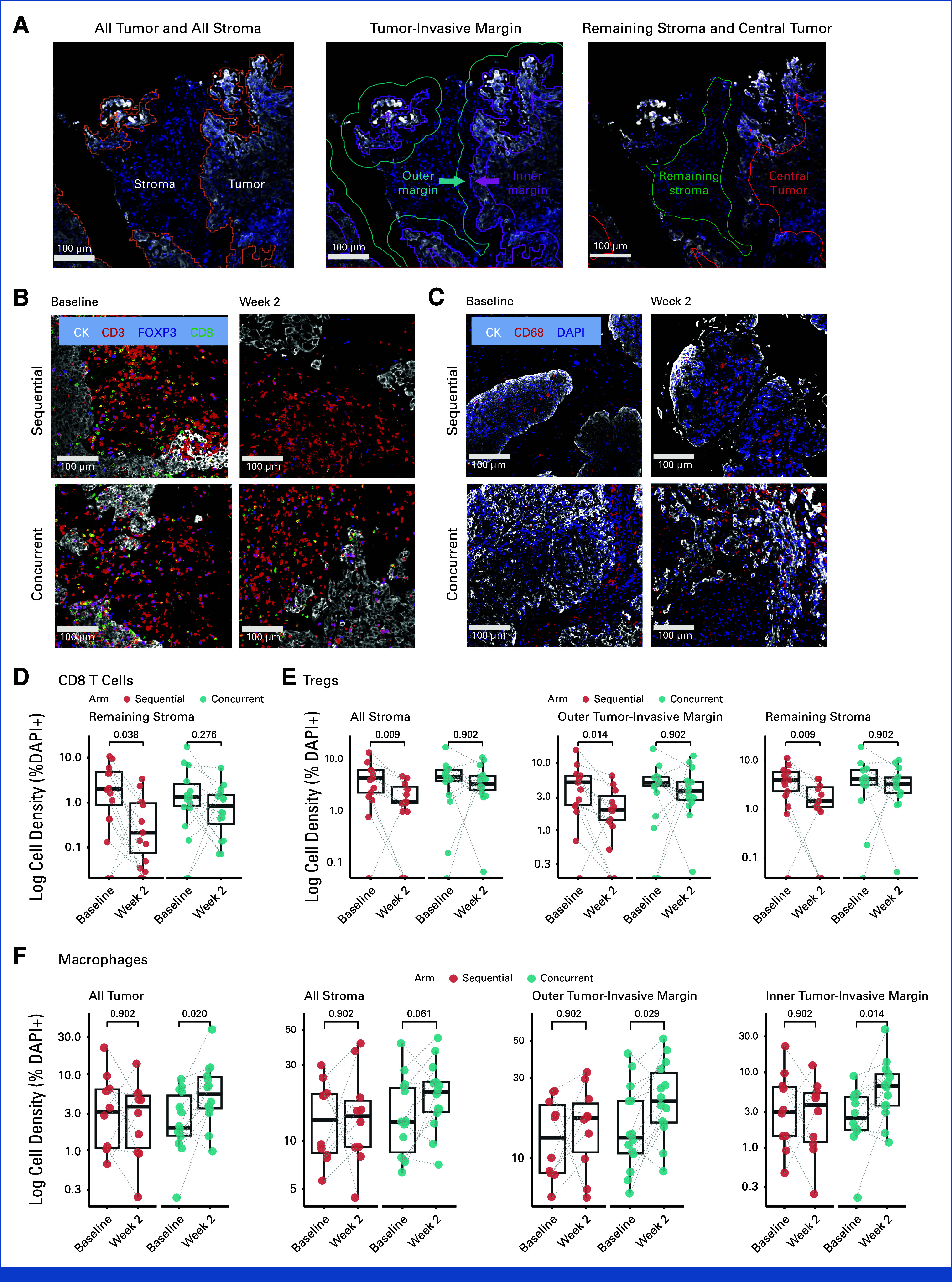
Immune cell densities in paired pretreatment and on-treatment biopsy samples by arm.
Expert Commentary
This trial provides the first randomized evidence that the sequence of immune checkpoint inhibition relative to CRT can substantially impact long-term locoregional control in high-risk HNSCC. The inferior LRC in the concurrent arm, despite an initial high response rate, suggests that immunosuppressive TME alterations induced by early pembrolizumab may counteract potential benefit. In contrast, deferring immunotherapy until after CRT may allow for the restoration of antitumor immunity, leveraging the immunogenic effects of radiation and chemotherapy. Importantly, both approaches were feasible and safe, but only the sequential arm achieved robust, durable control.
These findings challenge the prevailing trend of simply adding immunotherapy to standard CRT regimens and underscore the need for mechanistic and clinical studies optimizing the temporal integration of these modalities. Limitations include the relatively small sample size and the need for validation in larger, phase III trials. The observed differences in TME biology between arms offer a valuable foundation for future correlative studies and biomarker development.
Conclusion
In patients with locally advanced HNSCC, sequential pembrolizumab following chemoradiation achieved significantly superior long-term locoregional control compared to concurrent administration, with favorable trends in progression-free and overall survival. The trial highlights the critical importance of treatment sequencing and provides a rationale for further investigation of post-CRT immunotherapy strategies. These results may inform future guidelines and personalized approaches in the management of high-risk head and neck cancer.
References
1. Zandberg DP, Vujanovic L, Clump DA, et al. Randomized Phase II Study of Concurrent Versus Sequential Pembrolizumab in Combination With Chemoradiation in Locally Advanced Head and Neck Cancer. J Clin Oncol. 2025 Aug 10;43(23):2572-2582. doi: 10.1200/JCO-24-01580 IF: 41.9 Q1 . Epub 2025 May 27. PMID: 40424564 IF: 41.9 Q1 ; PMCID: PMC12316147 IF: 41.9 Q1 .2.