Highlight
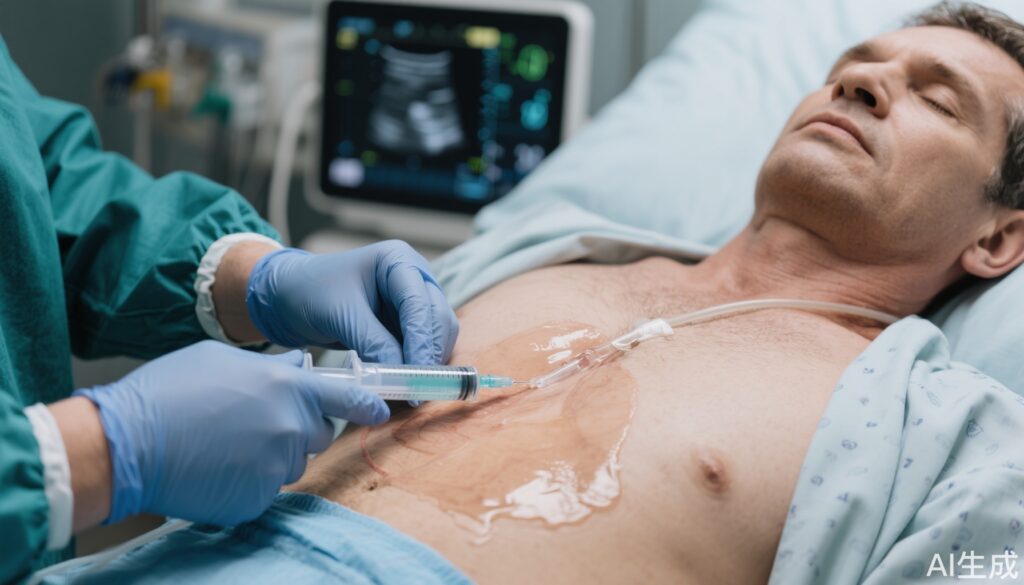
The TAP-IT trial evaluated the role of routine thoracentesis added to standard medical therapy in acute heart failure patients with moderate pleural effusion. No significant benefit was demonstrated in days alive out of hospital, mortality, or hospital stay length. Procedural complications were rare, supporting safety of thoracentesis but questioning its routine use.
Study Background and Disease Burden
Acute heart failure (AHF) is a prevalent clinical syndrome characterized by rapid onset or worsening of symptoms and signs of heart failure, often requiring urgent hospitalization. Often associated with pulmonary congestion, many patients also develop pleural effusions stemming from elevated left ventricular filling pressures and fluid overload. Such pleural effusions can contribute to dyspnea and impaired respiratory mechanics, posing an additional therapeutic challenge.
While urgent decongestive therapy is the cornerstone of AHF management, the role of thoracentesis for pleural effusions secondary to heart failure remains unclear. Current guidelines do not strongly recommend routine thoracentesis due to limited evidence. The TAP-IT trial aimed to address this clinical uncertainty by investigating if upfront therapeutic thoracentesis could improve clinical outcomes beyond standard medical therapy in AHF patients with sizeable—but not massive—pleural effusions.
Study Design
The TAP-IT trial was a multicenter, unblinded randomized controlled trial conducted from August 2021 to March 2024. It enrolled 135 patients hospitalized with acute heart failure defined by left ventricular ejection fraction ≤45% and moderate pleural effusion measurable on imaging. Patients with very large effusions occupying more than two-thirds of the hemithorax were excluded to maintain safety and homogeneity.
Participants were randomly assigned in a 1:1 ratio to either upfront ultrasound-guided pleural pigtail catheter thoracentesis plus standard medical therapy or standard medical therapy alone. The primary endpoint was the number of days alive and out of hospital during the 90-day follow-up period. Key secondary endpoints included length of index hospital admission and 90-day all-cause mortality. All analyses adhered to the intention-to-treat principle.
Key Findings
Among 135 randomized patients (median age 81 years, 33% female, median LVEF 25%), 68 underwent thoracentesis and 67 received standard therapy alone. The primary outcome—days alive out of hospital—showed a median of 84 days (IQR 77–86) in the thoracentesis group versus 82 days (IQR 73–86) in controls, a non-significant difference (P=0.42).
Mortality at 90 days was identical at 13% in both groups with no survival benefit from thoracentesis (P=0.90). Additionally, length of the index admission was similar between groups (median 5 days in control vs. 5 days in thoracentesis, P=0.69). Importantly, major complications related to thoracentesis were very rare, documented in only 1% of procedures, confirming procedural safety in this population.
These results indicate that adding routine thoracentesis for pleural effusions in AHF patients with mildly to moderately sized effusions does not enhance short-term clinical outcomes compared with medical therapy alone.
Expert Commentary
The TAP-IT trial offers important evidence for clinicians managing pleural effusions in acute heart failure. The absence of improvement in days alive out of hospital or mortality suggests that routine thoracentesis may not be necessary unless effusions are very large or causing significant symptoms refractory to medical therapy.
The low complication rate supports that when clinically indicated, ultrasound-guided thoracentesis remains a safe intervention. However, the overall neutral results question the routine upfront use of thoracentesis, emphasizing reliance on optimized heart failure pharmacotherapy for congestion relief.
Some limitations include the exclusion of patients with very large effusions, which represent a subgroup possibly more likely to benefit from drainage. Further studies could explore targeted thoracentesis in symptomatic relief in select patients or investigate longer-term outcomes beyond 90 days.
Conclusion
The TAP-IT trial demonstrated that in patients hospitalized for acute heart failure with moderate pleural effusion, a strategy of immediate thoracentesis plus standard medical therapy does not improve days alive out of hospital, mortality, or hospital stay duration compared to standard medical therapy alone. Thoracentesis was safe but without clinical benefit on these outcomes.
These findings support careful patient selection for thoracentesis and highlight the need for further research on personalized approaches to managing pleural effusion in heart failure. Meanwhile, optimizing medical therapy remains paramount in this population.
References
Glargaard S, Thomsen JH, Tuxen C, et al. A Randomized Controlled Trial of Thoracentesis in Acute Heart Failure. Circulation. 2025 Apr 22;151(16):1150-1161. doi: 10.1161/CIRCULATIONAHA.124.073521 . PMID: 40166829 ; PMCID: PMC12011436 .