Introduction
The management of severe symptomatic aortic stenosis (AS) has been transformed over the past decade by transcatheter aortic valve implantation (TAVI). Initially reserved for patients at prohibitive or high surgical risk, TAVI expanded rapidly as randomized trials showed comparable or superior short‑term outcomes versus surgical aortic valve replacement (SAVR) in intermediate and selected low‑risk populations. Landmark trials such as PARTNER, SURTAVI, PARTNER 3 and Evolut Low Risk shaped guideline updates (ACC/AHA, ESC/EACTS) and clinical practice.1,2
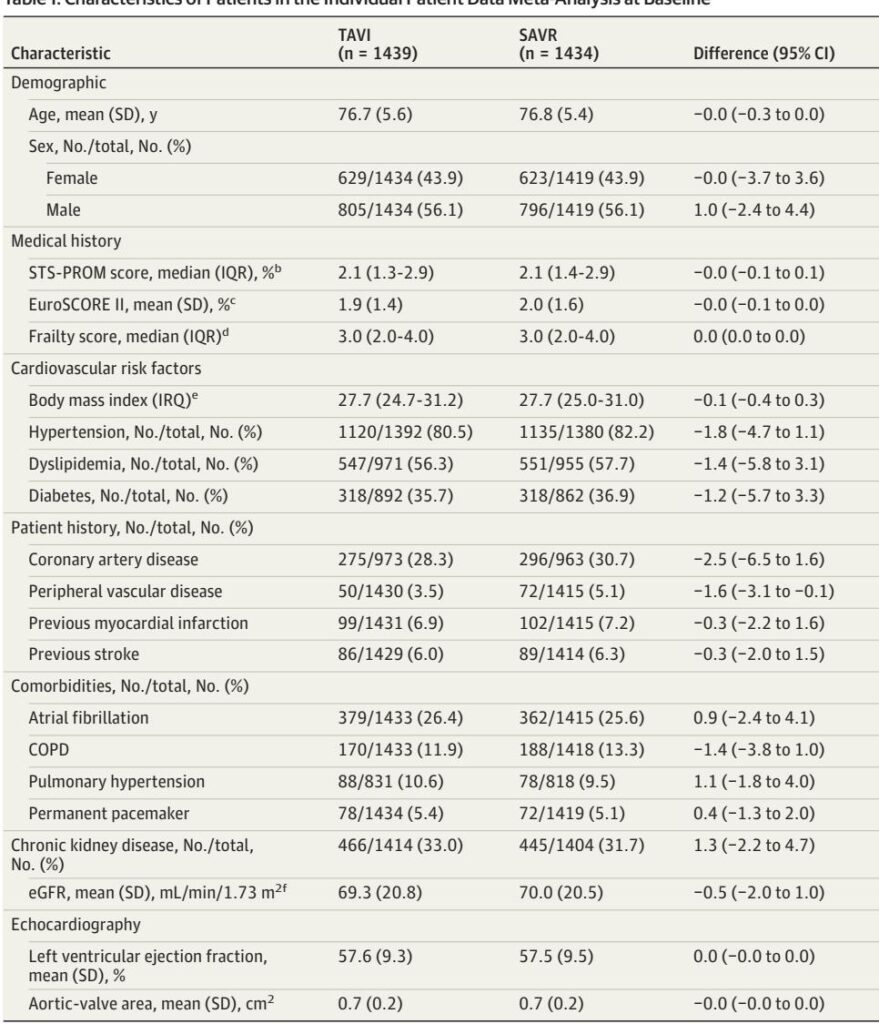
Yet randomized trials have important heterogeneity in design, valve types, patient selection and follow‑up. Investigator‑initiated “pragmatic” trials enroll broader, more representative patients; industry‑sponsored trials often apply narrow inclusion criteria and valve‑specific protocols. To reconcile differences and improve precision, Ludwig et al. performed an individual participant data (IPD) meta‑analysis of four investigator‑initiated randomized clinical trials (DEDICATE, NOTION, NOTION‑2, UK TAVI) and combined those results with aggregate data from four industry trials (PARTNER 2A, PARTNER 3, SURTAVI, Evolut Low Risk). The pooled analyses encompassed 8 trials and ~9,000 patients (IPD n=2,873; overall n≈9,069) and focused on 1‑year clinical outcomes.3
Why this analysis matters now: clinicians and guideline committees face increased pressure to apply TAVI to younger or lower‑risk patients. The IPD meta‑analysis provides patient‑level harmonization, allows consistent modeling of competing risks, and delivers more robust subgroup insights than single trials or standard aggregate meta‑analyses. The results should influence heart‑team decisions, informed consent discussions, and guideline updates—while highlighting gaps that require long‑term data.
New Meta‑analysis Highlights
– Primary finding: Across the investigator‑initiated trials, TAVI reduced the 1‑year composite of all‑cause death or any stroke versus SAVR (1‑stage IPD sHR 0.73; 95% CI, 0.56–0.95). A 2‑stage analysis combining IPD and aggregate data yielded a similar overall hazard ratio of 0.76 (95% CI, 0.60–0.97).3
– Death alone: In the IPD set, all‑cause death at 1 year was lower after TAVI (HR ~0.55; 95% CI, 0.38–0.81). This signal attenuated when industry trials were added, producing an overall HR of 0.80 that did not meet conventional statistical significance.3
– Stroke: No robust difference in any stroke (disabling + nondisabling) at 1 year was found across pooled datasets; event curves crossed around 6 months suggesting complex temporal dynamics.3
– Secondary signals: TAVI was associated with less new‑onset atrial fibrillation but more permanent pacemaker implantation and a higher rate of aortic‑valve reintervention at 1 year in pooled analyses—driven partly by earlier generation valves.3
– Subgroups: Benefit for the composite outcome was clearer in patients with STS‑PROM ≥2% and showed a trend for patients aged ≥75 years. Inclusion of bicuspid AS patients (NOTION‑2 cohort) attenuated TAVI benefit.3
Key takeaways for clinicians
– For many low‑ and intermediate‑risk patients with tricuspid severe symptomatic AS, contemporary evidence supports TAVI as an alternative—and in some settings a preferred—option at 1 year.
– Decisions should remain individualized: anatomy (including bicuspid valve), life expectancy, valve durability priorities and conduction disease risk are central to choice of therapy.
Updated Recommendations and Key Changes (Compared with Prior Guidance)
The meta‑analysis does not replace formal guideline statements, but it consolidates RCT evidence in a way that aligns with and strengthens recent guideline trends. Below is a synthesis of how the new evidence refines prior recommendations (ACC/AHA 2020; ESC/EACTS 2021)1,2:
– Prior position (ACC/AHA 2020; ESC/EACTS 2021): TAVI recommended as the preferred approach in older patients or those at high/prohibitive surgical risk; reasonable alternative in selected low‑risk patients depending on age, anatomy, and patient preference.1,2
– New evidence impact: The IPD meta‑analysis shows an early (1‑year) clinical advantage for TAVI on the composite of death or stroke in low–intermediate risk patients—supporting broader use of TAVI in those aged ≥65–75 years with tricuspid anatomy and suitable access.3
Table: Conceptual comparison (simplified)
– Population now better supported for TAVI: Low‑ to intermediate‑risk patients with tricuspid AS, especially STS‑PROM ≥2% or age ≥75.
– Populations requiring caution: Patients <70, those with bicuspid valves, complex root anatomy, or significant lifetime valve durability concerns—evidence limited or attenuated.3,5
Evidence driving the updates
– Multiple low‑risk randomized trials (PARTNER 3, Evolut Low Risk; 5‑year data) showed TAVI noninferiority for key clinical outcomes.7,8,22
– Investigator‑initiated, pragmatic RCTs (DEDICATE, UK TAVI, NOTION / NOTION‑2) capture more heterogenous “real‑world” patients; IPD pooling amplifies their insights by harmonizing endpoints and enabling subgroup analyses.3–5
Topic‑by‑Topic Recommendations and Practical Guidance
Below are clinician‑focused recommendations and operational details derived from the pooled evidence, trial protocols, and contemporary guideline principles.
1) Who qualifies: diagnostic and clinical criteria
– Indication: Severe symptomatic aortic stenosis meeting guideline thresholds (e.g., mean gradient ≥40 mmHg, aortic valve area ≤1.0 cm2 or indexed criteria) with symptomatic status consistent with heart‑team evaluation.1,2
– Surgical risk: Low to intermediate surgical risk (median STS‑PROM in pooled IPD ≈2.1%; range included STS‑PROM both <2% and ≥2%). The meta‑analysis shows a stronger composite benefit when STS‑PROM ≥2%.3
– Anatomy: Best evidence applies to tricuspid aortic valve anatomy. Bicuspid AS patients were largely excluded or formed small cohorts (NOTION‑2 bicuspid cohort attenuated benefit); caution applying TAVI broadly to bicuspid anatomy.3,5
Recommendation (practical): For patients aged ≥75 or STS‑PROM ≥2% with tricuspid anatomy and suitable vascular access, offer TAVI as a first‑line option after heart‑team evaluation (evidence quality: moderate for 1‑year composite; IPD GRADE assessment reported in the study).3
2) Heart‑team decision framework
– Mandatory multidisciplinary evaluation (cardiac surgeon, interventional cardiologist, imaging specialist, anesthesiologist, geriatric/frailty assessment when indicated).
– Shared decision‑making that integrates life expectancy, patient values (e.g., prioritizing recovery time over durability), anatomic suitability (annulus size, calcification, coronary height), and conduction disease risk.
3) Valve platform and access
– Valve selection should consider patient anatomy and operator experience. Balloon‑expandable and self‑expanding platforms have different conduction and paravalvular leak profiles. Recent trials included multiple device generations—improvements in newer devices likely reduce earlier device‑related complications.3,7,8
– Transfemoral access remains preferred when feasible due to lower invasiveness and faster recovery; alternative access routes reserved for unsuitable peripheral anatomy.
4) Conduction abnormalities and pacemaker planning
– TAVI carries higher rates of new permanent pacemaker implantation (PPI) versus SAVR (IPD: TAVI 15.4% vs SAVR 6.9%), driven mainly by self‑expanding prostheses and baseline conduction disease.3
Practical steps:
– Baseline ECG and careful evaluation for preexisting conduction disease (e.g., RBBB, AV block) prior to choosing valve type.
– Post‑TAVI rhythm monitoring for ≥48–72 hours, with a low threshold for electrophysiology consultation if progressive conduction disease.
– Inform patients about the higher PPI risk and implications for follow‑up and potential pacing dependency.
5) Stroke prevention and periprocedural cerebral protection
– The meta‑analysis did not show a consistent stroke reduction for TAVI vs SAVR at 1 year. Embolic protection device data are mixed; not yet standard of care for all patients.3
Recommendation: Follow institutional protocols for antithrombotic strategy and consider embolic protection selectively in patients at particularly high embolic risk or with heavy leaflet/annular calcium until stronger evidence or guideline endorsement exists.
6) Reintervention and valve durability
– The pooled analysis found higher 1‑year aortic‑valve reintervention rates after TAVI, influenced by older valve generations. Long‑term durability data beyond 5–10 years remain the key uncertainty for younger patients.3,20–23,25,26
Practical guidance:
– In patients with long life expectancy (<70 years), prioritize conversations about durability, potential need for future reinterventions (valve‑in‑valve strategies vs redo‑SAVR), and available long‑term trial evidence.
– Maintain structured echocardiographic surveillance (discharge, 30 days, 1 year, then annually or as clinically indicated) to detect early structural valve deterioration.
7) Antithrombotic strategy post‑procedure
– Antithrombotic regimens vary by trial and device; align with ACC/AHA or ESC guidance and individual bleeding/thrombotic risk. Current evidence favors single antiplatelet therapy post‑TAVI in patients without other indications for oral anticoagulation, but tailor to patient comorbidities (e.g., AF, prior thromboembolism).1,2
8) Follow‑up and surveillance
– Recommended practical follow‑up schedule: clinical review and ECG at discharge and 30 days; transthoracic echocardiogram at discharge, 30 days and 1 year; annual clinical and echo follow‑up thereafter. For any new symptoms (dyspnea, syncope), expedite imaging and cardiology review.
9) Special populations
– Bicuspid AS: Evidence limited and benefit attenuated in pooled data; treat cautiously—consider SAVR for many younger bicuspid patients, or TAVI only within experienced centers and after detailed imaging assessment.3,5
– Patients <70 years: Insufficient long‑term durability data to broadly recommend TAVI as default; shared decision‑making essential.
– Frail or comorbid patients: Pragmatic IITs included higher event rates, reflecting real‑world risk. Heart‑team decisions should weigh frailty, anticipated recovery and patient goals.
Expert Commentary and Insights
What the committee authors and other experts emphasize:
– Harmonized IPD strengthens confidence in short‑term advantages for TAVI across a broader, more representative population than industry trials alone. The early divergence in cumulative incidence favoring TAVI suggests reduced perioperative morbidity and mortality related to a less invasive approach.3
– Caveats raised by experts:
– Durability remains the chief limitation. Although 5‑ and 8–10‑year data from specific trials (NOTION, PARTNER 2A, SURTAVI, PARTNER 3, Evolut Low Risk) are reassuring in some respects, signals of late events have been observed and longer follow‑up with pooled IPD is needed.20–23,25,26
– Heterogeneity in device generations across trials complicates interpretation: earlier valves had different safety and durability profiles. Ongoing surveillance of contemporary devices is required.
– Bicuspid anatomy and very young patients remain controversial—trial evidence is sparse and mixed; many experts recommend SAVR for younger bicuspid patients absent compelling device‑specific evidence.
Areas of consensus: TAVI is an appropriate option for many low‑ to intermediate‑risk patients after heart‑team assessment; shared decision‑making and individualized risk‑benefit discussion are essential.3
Areas of controversy: Long‑term valve durability and the optimal management strategy for younger patients and those with bicuspid valves; strategies to reduce conduction disease after TAVI remain an active research area.27,28
Practical Implications for Clinical Practice
– Patient selection will shift: Expect increasing TAVI volumes for older and many intermediate‑risk patients, with continued SAVR for younger patients, complex anatomy, or when durability is the top priority.
– Heart‑team workflows should be reinforced: The multidisciplinary approach is not optional—outcomes depend on imaging expertise, device selection, and surgical backup.
– Informed consent should expand to address:
– The demonstrated 1‑year reduction in death or stroke with TAVI in pooled IPD;3
– Increased PPI risk with TAVI and potential implications;3
– Uncertainties about longer‑term durability and reintervention.
– Systems planning: Increased TAVI adoption requires infrastructure for device supply, operator training, imaging capacity, and structured follow‑up clinics.
Fictional Vignette: Applying the Evidence
John Smith, 78, symptomatic with exertional dyspnea; echocardiogram shows severe tricuspid aortic stenosis (AVA 0.8 cm2), STS‑PROM 2.4%, moderate chronic kidney disease (eGFR 50). After heart‑team review comparing risks and values, and given suitable transfemoral access and John’s strong preference to avoid sternotomy and a quicker recovery, the team offers TAVI. The discussion includes the IPD meta‑analysis outcome (lower 1‑year risk of death or stroke with TAVI), the higher pacemaker risk (and plans for rhythm monitoring), and the need for routine echocardiographic surveillance. This shared decision respects patient preference while grounding the choice in the best available pooled evidence.3
What We Still Need—Research and Data Gaps
– Long‑term durability: 5–10+ year pooled IPD analyses to define structural valve deterioration, late mortality and reintervention risk across valve platforms and patient subgroups.20–23,25,26
– Bicuspid valves: randomized data and device optimization for bicuspid anatomy.
– Younger patients: trials or registries focused on patients <70 years, integrating quality of life and lifetime reintervention planning.
– Conduction mitigation strategies to reduce post‑TAVI pacemaker rates, including procedural techniques, valve selection and device design.27,28
– Real‑world registry linkage with IPD to validate trial findings in unselected populations and across diverse health systems.
Bottom line
The IPD meta‑analysis consolidates randomized evidence that, at 1 year, TAVI is associated with lower rates of the composite outcome of death or stroke compared with SAVR in many low‑ and intermediate‑risk patients with tricuspid severe symptomatic AS. While the findings strengthen the case for broader use of TAVI, equipoise remains for younger patients and those with bicuspid anatomy because of limited long‑term durability data. Shared decision‑making, formal heart‑team evaluations, and vigilant long‑term surveillance remain essential as the field matures and longer‑term pooled data become available.
References
1. Otto CM, Nishimura RA, Bonow RO, et al. 2020 ACC/AHA Guideline for the Management of Patients With Valvular Heart Disease. Circulation. 2021;143(5):e35–e71. doi:10.1161/CIR.0000000000000932 IF: 38.6 Q1 2. Vahanian A, Beyersdorf F, Praz F, et al; ESC/EACTS Scientific Document Group. 2021 ESC/EACTS guidelines for the management of valvular heart disease. Eur Heart J. 2022;43(7):561–632. doi:10.1093/eurheartj/ehab395 IF: 35.6 Q1 3. Ludwig S, Klimek M, Bay B, et al. Transcatheter or Surgical Treatment of Patients With Aortic Stenosis at Low to Intermediate Risk: An Individual Participant Data Meta‑Analysis. JAMA (published 2025). (Primary paper summarized.)
4. Toff WD, Hildick‑Smith D, Kovac J, et al; UK TAVI Trial Investigators. Effect of TAVI vs SAVR on all‑cause mortality in patients with aortic stenosis: randomized trial. JAMA. 2022;327(19):1875–1887. doi:10.1001/jama.2022.5776 IF: 55.0 Q1 5. Jørgensen TH, Thyregod HGH, Savontaus M, et al; NOTION‑2 investigators. NOTION‑2 trial. Eur Heart J. 2024;45(37):3804–3814. doi:10.1093/eurheartj/ehae331 IF: 35.6 Q1 6. Leon MB, Smith CR, Mack MJ, et al; PARTNER 2 Investigators. N Engl J Med. 2016;374(17):1609–1620. doi:10.1056/NEJMoa1514616 IF: 78.5 Q1 7. Mack MJ, Leon MB, Thourani VH, et al; PARTNER 3 Investigators. N Engl J Med. 2019;380(18):1695–1705. doi:10.1056/NEJMoa1814052 IF: 78.5 Q1 8. Popma JJ, Deeb GM, Yakubov SJ, et al; Evolut Low Risk Trial Investigators. N Engl J Med. 2019;380(18):1706–1715. doi:10.1056/NEJMoa1816885 IF: 78.5 Q1 9. Reardon MJ, Van Mieghem NM, Popma JJ, et al; SURTAVI Investigators. N Engl J Med. 2017;376(14):1321–1331. doi:10.1056/NEJMoa1700456 IF: 78.5 Q1 10. Thyregod HG, Steinbrüchel DA, Ihlemann N, et al. NOTION 1‑year results. J Am Coll Cardiol. 2015;65(20):2184–2194. doi:10.1016/j.jacc.2015.03.014 IF: 22.3 Q1 11. Guyot P, Ades AE, Ouwens MJ, Welton NJ. Reconstructing data from published Kaplan–Meier curves. BMC Med Res Methodol. 2012;12:9. doi:10.1186/1471-2288-12-9 IF: 3.4 Q1 12. Burke DL, Ensor J, Riley RD. One‑stage and two‑stage IPD meta‑analysis approaches. Stat Med. 2017;36(5):855–875. doi:10.1002/sim.7141 IF: 1.8 Q1 13. Katsahian S, Resche‑Rigon M, Chevret S, Porcher R. Mixed proportional hazards for subdistribution. Stat Med. 2006;25(24):4267–4278. doi:10.1002/sim.2684 IF: 1.8 Q1 14. Rueten‑Budde AJ, Putter H, Fiocco M. Investigating hospital heterogeneity with competing risks frailty model. Stat Med. 2019;38(2):269–288. doi:10.1002/sim.8002 IF: 1.8 Q1 15. Putter H, Fiocco M, Geskus RB. Competing risks and multi‑state models tutorial. Stat Med. 2007;26(11):2389–2430. doi:10.1002/sim.2712 IF: 1.8 Q1 16. Zhang X, Zhang MJ, Fine J. Proportional hazards regression for the subdistribution. Stat Med. 2011;30(16):1933–1951. doi:10.1002/sim.4264 IF: 1.8 Q1 17. Watkins C, Bennett I. Combining counts with hazard ratios. Res Synth Methods. 2018;9(3):352–360. doi:10.1002/jrsm.1301 IF: 6.1 Q1 18. Higgins JPT, Thomas J, Chandler J, et al. Cochrane Handbook for Systematic Reviews of Interventions version 6.5 (2024).
19. Guyatt GH, Oxman AD, Vist GE, et al; GRADE Working Group. BMJ. 2008;336(7650):924–926. doi:10.1136/bmj.39489.470347.AD IF: 42.7 Q1 20. Makkar RR, Thourani VH, Mack MJ, et al; PARTNER 2 5‑year outcomes. N Engl J Med. 2020;382(9):799–809. doi:10.1056/NEJMoa1910555 IF: 78.5 Q1 21. Van Mieghem NM, Deeb GM, Søndergaard L, et al; SURTAVI 5‑year outcomes. JAMA Cardiol. 2022;7(10):1000–1008. doi:10.1001/jamacardio.2022.2695 IF: 14.1 Q1 22. Mack MJ, Leon MB, Thourani VH, et al; PARTNER 3 5‑year outcomes. N Engl J Med. 2023;389(21):1949–1960. doi:10.1056/NEJMoa2307447 IF: 78.5 Q1 23. Forrest JK, Yakubov SJ, Deeb GM, et al. Low Risk 5‑Year outcomes. J Am Coll Cardiol. 2025;85(15):1523–1532. doi:10.1016/j.jacc.2025.03.004 IF: 22.3 Q1 24. Doenst T, Gregg AC, Kirov H, et al. Meta‑analysis of low‑risk randomized trials with 5‑year follow up. Eur J Cardiothorac Surg. 2025;67(7):ezaf215. doi:10.1093/ejcts/ezaf215 IF: 3.0 Q1 25. Jørgensen TH, Thyregod HGH, Ihlemann N, et al. Eight‑year outcomes NOTION. Eur Heart J. 2021;42(30):2912–2919. doi:10.1093/eurheartj/ehab375 IF: 35.6 Q1 26. Thyregod HGH, Jørgensen TH, Ihlemann N, et al. NOTION 10‑year outcomes. Eur Heart J. 2024;45(13):1116–1124. doi:10.1093/eurheartj/ehae043 IF: 35.6 Q1 27. Bisson A, Bodin A, Herbert J, et al. Pacemaker implantation after TAVI: JAHA. 2020;9(9):e015896. doi:10.1161/JAHA.120.015896 IF: 5.3 Q1 28. Ullah W, Zahid S, Zaidi SR, et al. Predictors of PPI after TAVI—a systematic review. JAHA. 2021;10(14):e020906. doi:10.1161/JAHA.121.020906 IF: 5.3 Q1