Posted inHematology-Oncology news
Fractionated Dosing of Varnimcabtagene Autoleucel Delivers Deep Remissions and Enhanced Safety in Adult B-ALL
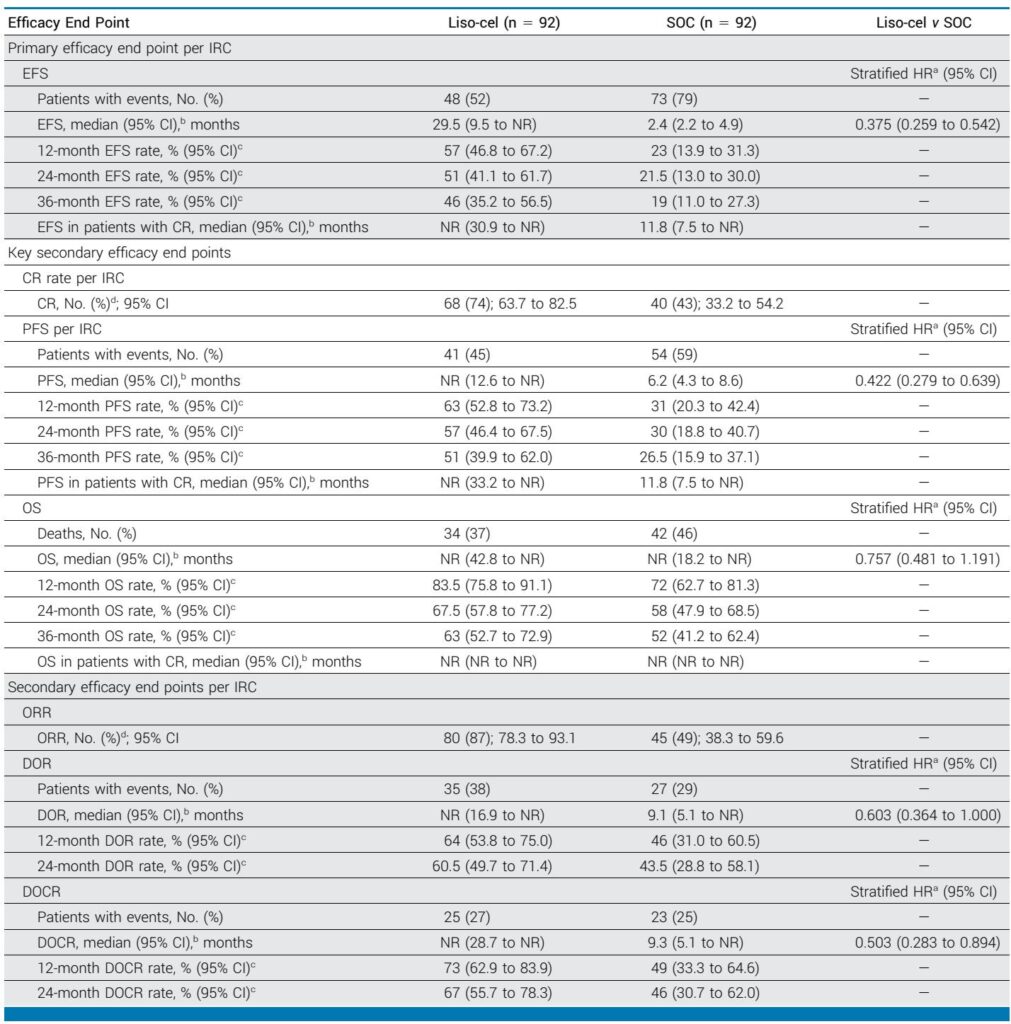
The CART19-BE-02 phase 2 trial demonstrates that varnimcabtagene autoleucel, utilizing a unique fractionated dose escalation strategy, achieves an 84.4% MRD-negative complete response rate in adults with relapsed or refractory B-ALL while significantly reducing the incidence of severe neurotoxicity and cytokine release syndrome.