Highlight
– Routine Helicobacter pylori (H. pylori) screening during hospitalization for myocardial infarction (MI) did not significantly lower upper gastrointestinal bleeding (UGIB) incidence.
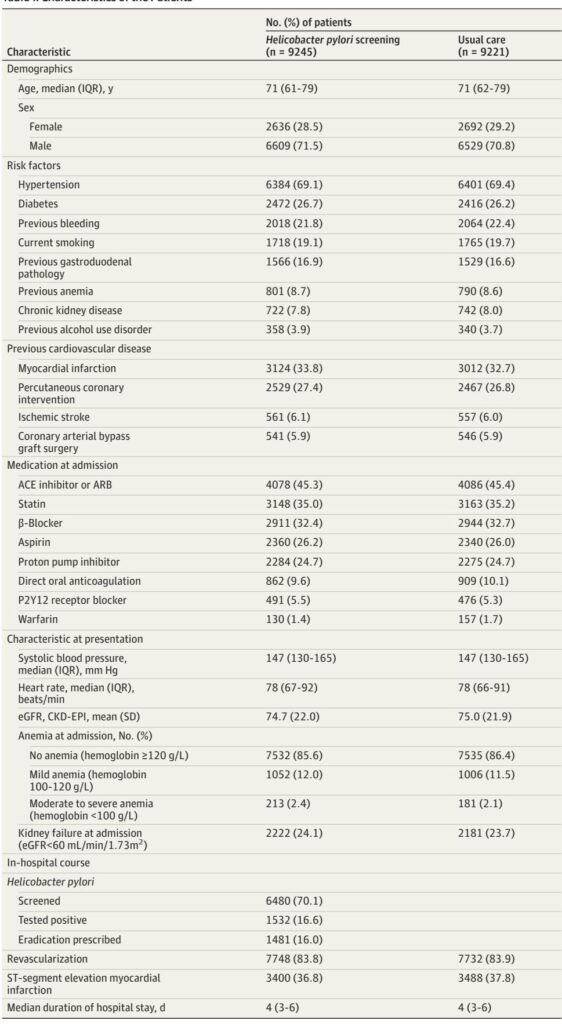
– About 70% of patients were screened, with a 23.6% infection positivity rate.
– Subgroup analyses suggest potential benefit of screening in patients with anemia or kidney failure.
– Current evidence does not support universal H. pylori screening after MI, but targeted approaches may be warranted.
Background
Despite advances in post-acute myocardial infarction (MI) care, including potent antiplatelet therapy and early revascularization, bleeding complications remain a clinical challenge. Upper gastrointestinal bleeding (UGIB) is the predominant bleeding site post-MI and is associated with increased mortality and cardiovascular events. Proton pump inhibitors (PPIs) reduce UGIB risk but their routine long-term use remains controversial. Chronic active gastritis caused by H. pylori is prevalent among cardiovascular patients and exacerbates UGIB risk, especially when combined with antithrombotic therapy. While gastroenterology guidelines recommend H. pylori screening for long-term aspirin users, cardiology guidelines do not currently endorse this practice. The HELP-MI SWEDEHEART trial addresses the clinical question of whether routine H. pylori screening during MI hospitalization reduces UGIB events.
Study Design
HELP-MI SWEDEHEART was a nationwide, open-label, two-period, two-sequence cluster randomized crossover trial conducted across 35 Swedish hospitals grouped into 18 clusters. Between November 17, 2021, and January 17, 2024, clusters alternated between 1 year of routine H. pylori screening using the 13C-urea breath test and 1 year of usual care, with a 2-month washout period before crossover. The study population included adults discharged alive with type 1 MI registered in SWEDEHEART and followed until January 17, 2025.
The primary endpoint was incidence of upper gastrointestinal bleeding, analyzed in an intention-to-treat framework using a negative binomial model. Secondary endpoints included composite clinical outcomes (net adverse clinical events, cardiovascular events) and all-cause and cardiovascular mortality.
Key Findings
Among 18,466 patients (median age 71 years; 71% male), 9245 underwent routine screening, with 70% compliance and 23.6% testing positive for H. pylori. Nearly all patients testing positive (96.6%) received eradication therapy at physicians’ discretion.
Over a median follow-up of 1.9 years, 299 UGIB events occurred in the screening group (incidence rate 16.8 per 1000 person-years) versus 336 in usual care (19.2 per 1000 person-years). Routine screening did not significantly reduce UGIB risk (rate ratio 0.90; 95% CI, 0.77–1.05; P = .18).
No significant differences emerged in secondary clinical endpoints or mortality. Subgroup analyses revealed a heterogeneous effect where patients with mild anemia (hemoglobin 100–120 g/L; RR 0.64) and moderate to severe anemia (hemoglobin <100 g/L; RR 0.44) demonstrated notable reductions in UGIB risk with screening (P for interaction = .03). Similarly, patients with kidney failure (eGFR <60 mL/min/1.73 m2) benefited (RR 0.75) compared to those with preserved renal function. Notably, patients discharged without PPI treatment had a significant UGIB risk reduction following screening.
Per-protocol analyses suggested greater risk reduction among screened patients testing positive and receiving eradication therapy, though these findings did not reach statistical significance.
Expert Commentary
The HELP-MI SWEDEHEART trial represents a robust, pragmatic evaluation of routine H. pylori screening post-MI in a large, unselected population, leveraging high-quality nationwide registries. The cluster randomized crossover design enhanced internal validity while reflecting real-world implementation.
The overall null effect may be influenced by several factors: lower-than-anticipated H. pylori prevalence (23.6% vs expected 30%), 70% screening adherence, and widespread PPI use (~25% at admission, ~50% at discharge) possibly confounding test accuracy and bleeding risk. The urea breath test, although reliable, can yield false negatives with PPI use. The observed benefit in anemic and renal impairment subpopulations underscores the potential for risk-stratified screening approaches.
Limitations include reliance on ICD coding without central adjudication, potential underestimation of intervention effect due to incomplete screening adherence, and lack of systematic post-discharge follow-up on eradication success. The crossover design posed risk of period effects, mitigated by a washout interval.
These findings complement prior smaller studies such as the HEAT trial, which demonstrated transient benefit with H. pylori eradication in aspirin users but did not address the broader acute MI population receiving potent antithrombotic regimens.
Conclusion
Routine H. pylori screening for all patients hospitalized with acute myocardial infarction does not significantly reduce upper gastrointestinal bleeding incidence. Nonetheless, selected high-risk groups such as those with baseline anemia or renal impairment may derive benefit from targeted screening and eradication strategies. Future guidelines might consider integrating risk stratification tools to optimize bleeding prevention. These results caution against universal H. pylori screening and underscore the need for individualized patient assessment in post-MI management.
References
Hofmann R, James S, Sundqvist MO, Wärme J, Angerås O, Alfredsson J, Erlinge D, Arefalk G, Arstad G, Blomberg S, Fröbert O, Hambraeus K, Hellström PM, Lauermann J, Lidin M, Lindhagen L, Mourtzinis G, Schoede C, Thunström E, Voldberg B, Wagner H, Östlund O, Jernberg T, Bäck M. Helicobacter pylori Screening After Acute Myocardial Infarction: The Cluster Randomized Crossover HELP-MI SWEDEHEART Trial. JAMA. 2025 Sep 1. doi: 10.1001/jama.2025.15047. Epub ahead of print. PMID: 40887995.