Introduction
Stress-related psychiatric disorders such as major depressive disorder (MDD) and post-traumatic stress disorder (PTSD) continue to cause significant disability worldwide. These conditions are often resistant to current treatments, with about one-third of affected adults not achieving remission. Recent research underscores the critical role of the interplay between the peripheral immune system and the central nervous system (CNS) in the pathology of these disorders. In particular, stress triggers increased production and circulation of proinflammatory immune cells and cytokines, contributing to disease progression.
Peripheral Immune-to-CNS Communication in Stress
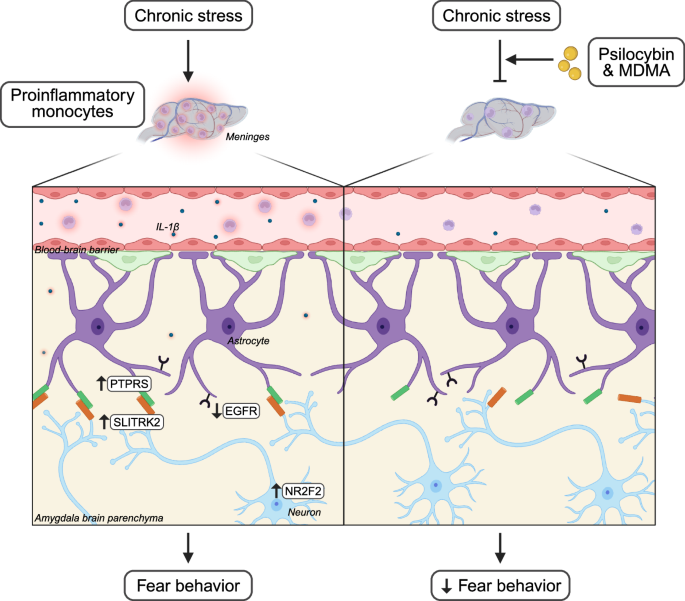
A pivotal study by Chung et al. identifies a novel communication pathway between peripheral immune cells and the CNS that is modulated by psychedelics. Stress increases circulating monocytes, which migrate to brain regions including neurovascular and meningeal compartments. Chronic restraint stress in rodent models induces fear behavior alongside elevations in immune cells such as monocytes within the meninges and increased proinflammatory cytokines like interleukin-1β (IL-1β) in the bloodstream.
Origins and Effects of Meningeal Monocytes
Using transgenic mice expressing photoconvertible fluorescent proteins, Chung and colleagues traced the origins of meningeal monocytes. Contrary to expectations, these immune cells arise not exclusively from the spleen but possibly from multiple reserves. Importantly, these meningeal monocytes release IL-1β directly into the brain parenchyma, facilitating direct immune-to-brain signaling.
Astrocytes and Neuronal Interactions in the Amygdala
The team employed single-cell RNA sequencing in transgenic mice with fluorescent astrocytes to identify a subpopulation responsive to stress. These astrocytes showed reduced epidermal growth factor receptor (EGFR) expression, a molecule previously linked to anti-inflammatory pathways.
Targeting EGFR in amygdala astrocytes using CRISPR-Cas9 revealed that EGFR downregulation heightened fear behaviors and activated inflammatory pathways such as NF-κB, including upregulation of protein tyrosine phosphatase receptor type S (Ptprs). This suggests EGFR-positive astrocytes normally act to suppress inflammation and stress-related behavioral responses.
Astrocyte-Neuron Communication and Fear Behavior
Further investigation demonstrated that astrocytic inflammation alters neuronal gene expression. Neurons showed increased Slitrk2, a receptor binding partner of PTPRS, and changes in the transcription factor nuclear receptor subfamily 2 group F member 2 (NR2F2), which is critical for stress-induced fear. Knockdown of NR2F2 reduced fear responses in vivo. Spatial transcriptomics confirmed that neurons expressing NR2F2 are in close proximity to EGFR-low astrocytes in the basolateral amygdala (BLA), highlighting the importance of astrocyte-neuron crosstalk in stress susceptibility.
Psychedelics Modulate Neuroimmune Communication
Crucially, psychedelics such as psilocybin and 3,4-methylenedioxymethamphetamine (MDMA) disrupt this inflammatory neuroimmune signaling. Administered after stress exposure, these compounds reduced fear behavior and decreased recruitment of proinflammatory immune cells to the meninges. In vitro exposure of immune cells to these psychedelics diminished their inflammatory profile; this effect was reversed by antagonists of serotonin 5-HT2 receptors, indicating that psychedelic action involves direct modulation of immune cell serotonin receptors.
Interactions between the immune system and CNS following chronic stress influence fear behavior.
Complexities and Additional Mechanisms
Other meninges cell types also express 5-HT2 receptors, and given the vasoconstrictive properties of psychedelics, the authors explored whether vascular effects contribute to antidepressant efficacy. They found that combining psychedelics with vasodilators negated reductions in immune cell recruitment to the meninges, suggesting complex interactions influence therapeutic outcomes.
Human Relevance and Translational Potential
To assess conservation in humans, single-nuclei RNA sequencing of the amygdala from MDD patients revealed a similar expansion of astrocyte subpopulations with downregulated EGFR signaling and increased excitatory neurons regulated by NR2F2. In vitro, human monocytes exposed to inflammatory stimuli combined with psychedelics showed reduced inflammatory profiles, mirroring rodent data and supporting translational relevance.
Conclusions and Future Directions
Psychedelics represent promising therapeutic agents for stress-related psychiatric disorders. While previous studies focused on neural circuitry and behavior, Chung et al.’s findings highlight the importance of neuroimmune interactions and their modulation by psychedelics. This expands understanding of how these compounds exert therapeutic effects and opens avenues for targeted drug development aimed at refining neuroimmune communication to alleviate stress-induced fear and anxiety.
Ongoing research into the neuroimmune axis will further enable development of novel treatments to improve outcomes for patients suffering from debilitating psychiatric conditions associated with stress.
Reference
Alvarez J, Russo SJ. Psychedelics target neuroimmune interactions to limit fear. Cell Res. 2025 Jul 25. doi: 10.1038/s41422-025-01154-z IF: 25.9 Q1 .
Shao LX, Liao C, Davoudian PA, Savalia NK, Jiang Q, Wojtasiewicz C, Tan D, Nothnagel JD, Liu RJ, Woodburn SC, Bilash OM, Kim H, Che A, Kwan AC. Psilocybin’s lasting action requires pyramidal cell types and 5-HT2A receptors. Nature. 2025 Jun;642(8067):411-420. doi: 10.1038/s41586-025-08813-6.