Highlight
- Pitolisant, a histamine-3 inverse agonist stimulatory agent, was tested as adjunctive therapy in refractory restless legs syndrome (RLS).
- In a small open-label trial, pitolisant significantly improved International RLS Rating Scale scores and daytime sleepiness scales over 8 weeks.
- Improvement was more notable in daytime consequences such as sleepiness rather than core RLS symptoms.
- Adverse events were generally mild, with insomnia being most frequent; pitolisant was overall well tolerated.
Study Background and Disease Burden
Restless legs syndrome (RLS) is a common sensorimotor neurological disorder characterized by an urge to move the legs accompanied by uncomfortable sensations, mostly during periods of rest and in the evening or night. RLS adversely affects sleep quality and daytime functioning, contributing significantly to patient morbidity. Standard treatments, including iron supplementation, dopaminergic agents, gabapentinoids, and opioids, alleviate symptoms but often become insufficient over time, leading to refractory RLS.
Interestingly, sedating antihistamines that cross the blood-brain barrier exacerbate RLS symptoms, highlighting the complex relationship between histaminergic neurotransmission and RLS pathophysiology. Pitolisant, an inverse agonist of the histamine-3 receptor (H3R), enhances central nervous system histamine release and is approved to treat narcolepsy characterized by excessive daytime sleepiness. Given pitolisant’s stimulant properties and influence on CNS histamine pathways, there is a scientific rationale to explore its potential benefit as adjunct therapy in refractory RLS, particularly targeting daytime symptoms and fatigue.
Study Design
This was an open-label pilot study enrolling 21 patients with refractory RLS inadequately managed by at least one standard RLS medication (dopaminergics, gabapentinoids, or opioids). The study population had a mean age of 67.6 years, predominantly male, with 76% reporting a family history of RLS, and many receiving dual therapy.
Pitolisant was titrated to a maximum dose of 35.6 mg, administered in the morning, over 8 weeks while other RLS treatments remained stable. The primary efficacy endpoint was change in the International Restless Legs Syndrome Rating Scale (IRLSRS) at Week 8. Secondary endpoints included assessments of fatigue, daytime sleepiness (Epworth Sleepiness Scale), cognition, and depression. Thereafter, patients continued pitolisant for an additional 8 weeks with allowed modifications to concomitant RLS treatments to monitor longer-term tolerability and effect.
Key Findings
Of the 21 enrolled subjects, 19 completed the initial 8 weeks. Two withdrew—one due to adverse effects including worsened ADHD/irritability and the other was lost to follow-up. Two patients did not reach the full dose.
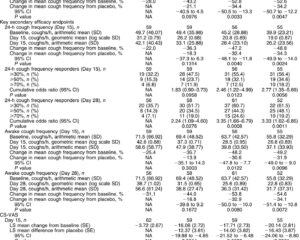
At Week 8, the mean IRLSRS score improved significantly from 24.5 to 17.2 (p < 0.001). A total of 8 out of 19 patients reported marked or very marked subjective improvement. Secondary scales such as the RLS-6 symptom scale also improved (p=0.005), and Epworth Sleepiness Scale showed significant reduction of daytime sleepiness (p < 0.01). Measures of fatigue, cognition, and depressive symptoms remained statistically unchanged.
Regarding safety, nine patients reported adverse events, the most common being insomnia in 6 subjects. No serious adverse events were reported, and pitolisant was generally well tolerated.
These findings suggest that pitolisant may exert a beneficial effect, preferentially improving daytime functional impairments and sleepiness rather than robustly reducing the core sensations and urges defining RLS.
Expert Commentary
The pilot study by Ondo WG provides important preliminary clinical insights into the utility of pitolisant as adjunct therapy for refractory RLS. The improvement in IRLSRS scores and daytime sleepiness scales supports the premise that stimulating CNS histaminergic pathways could ameliorate consequences of RLS beyond direct sensory symptoms.
The open-label design limits definitive conclusions due to the absence of placebo control and small sample size. The majority of patients were elderly, with multiple concurrent RLS medications, making generalizability uncertain. Insomnia as an adverse event aligns with the stimulant profile of pitolisant and warrants close monitoring in future trials.
From a mechanistic standpoint, the H3 receptor acts as an autoreceptor modulating histamine and other neurotransmitter release (including dopamine and acetylcholine). By inverse agonism at H3R, pitolisant’s enhancement of CNS histamine release may counteract the sedative effects associated with antihistamines and potentially improve vigilance and sleep architecture disrupted by RLS. This could explain the more pronounced improvement in daytime outcomes compared to nocturnal core symptoms.
The findings warrant larger, randomized, placebo-controlled trials to confirm efficacy, safety, optimal dosing, and to delineate patient subgroups most likely to benefit. Further studies could also explore pitolisant’s impact on RLS-related sleep fragmentation, quality of life, and long-term medication adherence.
Conclusion
This first-in-human pilot study demonstrates that pitolisant, a CNS-penetrant histamine-3 inverse agonist approved for narcolepsy, is a promising adjunctive therapy for refractory restless legs syndrome. It is generally well tolerated and leads to significant improvements in RLS symptom severity and daytime sleepiness. The differential improvement favoring daytime consequences over core nocturnal symptoms highlights a novel therapeutic angle targeting daytime dysfunction associated with RLS.
Given the study limitations, controlled trials are justified to validate these preliminary findings. If successful, pitolisant could become an important therapeutic option addressing unmet needs in refractory RLS patients, adding a new dimension by modulating histaminergic neurotransmission.
References
Ondo WG. Pilot study of Pitolisant, a histamine-3 inverse agonist, as adjunct therapy for refractory restless legs syndrome. Sleep Med. 2025 Sep;133:106634. doi: 10.1016/j.sleep.2025.106634. Epub 2025 Jun 10. PMID: 40554387.
Allen RP, Picchietti DL, Garcia-Borreguero D, et al. Restless legs syndrome/Willis-Ekbom disease diagnostic criteria: updated International Restless Legs Syndrome Study Group (IRLSSG) consensus criteria – history, rationale, description, and significance. Sleep Med. 2014 Aug;15(8):860-873. doi:10.1016/j.sleep.2014.03.025.
Dauvilliers Y, Bassetti CLA, Lammers GJ, et al. Pitolisant versus placebo for daytime sleepiness in narcolepsy: a phase 3, double-blind, randomised trial. Lancet Neurol. 2013 Apr;12(4):396-405. doi:10.1016/S1474-4422(13)70061-7.
Bassetti CL, Schwartz S. Histamine and sleep disorders. Sleep Med. 2023 Jan;103:118-132. doi:10.1016/j.sleep.2023.01.008.