Highlight
- Switching from nucleo(s)tide analogue (NUC) therapy to pegylated interferon alpha-2a (PegIFN-α-2a) significantly reduces virological relapse in HBeAg-negative chronic hepatitis B patients.
- Patients receiving PegIFN-α-2a demonstrated higher hepatitis B surface antigen (HBsAg) loss rates than those who stopped NUC therapy alone.
- The study offers an optimized treatment strategy for managing NUC discontinuation to reduce relapse and improve long-term viral control.
Study Background and Disease Burden
Chronic hepatitis B virus (HBV) infection remains a global public health challenge, leading to significant morbidity and mortality from cirrhosis and hepatocellular carcinoma. Current antiviral management principally involves prolonged treatment with nucleo(s)tide analogues (NUCs), which effectively suppress HBV replication. However, sustained virological control often requires indefinite therapy because cessation can precipitate high rates of virological relapse and clinical flares, especially in HBeAg-negative patients. Such relapses impose risks including hepatic decompensation and undermine efforts toward functional cure, characterized by the loss of hepatitis B surface antigen (HBsAg).
Pegylated interferon alpha-2a (PegIFN-α-2a), with its dual antiviral and immunomodulatory mechanisms, is an alternative therapy with reported potential to induce higher rates of HBsAg loss. Yet, its role as a consolidation treatment after NUC cessation, particularly to reduce relapse risk and promote viral clearance in HBeAg-negative patients, has been unclear. This unmet clinical need prompted the randomized controlled trial (NCT02594293) discussed here, focused on evaluating whether switching patients from long-term NUC therapy to PegIFN-α-2a for 48 weeks could optimize outcomes after treatment discontinuation.
Study Design
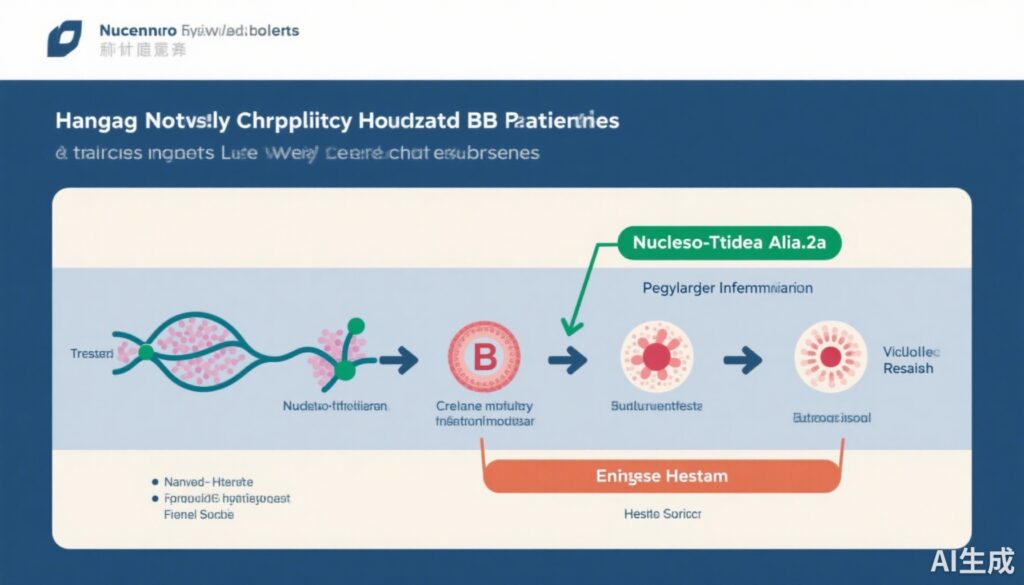
This multicenter, randomized-controlled clinical trial enrolled 180 non-cirrhotic patients with HBeAg-negative chronic hepatitis B who had been receiving continuous NUC therapy for at least 2.5 years with undetectable or very low HBV DNA (<60 IU/mL). Patients were randomized 1:1 into two arms:
1. NUC Cessation Group (n=90): Patients discontinued NUC therapy without further antiviral intervention.
2. PegIFN-α-2a Monotherapy Group (n=90): Patients stopped NUCs and immediately transitioned to PegIFN-α-2a for 48 weeks.
The primary endpoint was the cumulative virological relapse rate, defined by reappearance of HBV DNA above specified thresholds, measured up to 96 weeks from baseline. Patients were closely followed for virological, clinical, and serological markers including HBsAg levels and clinical relapse indicators.
Key Findings
Intention-to-treat analysis revealed a pronounced effect of PegIFN-α-2a in reducing relapse risk:
– Cumulative Virological Relapse Rates at Week 96: 20.8% in the PegIFN-α-2a group versus 53.6% in the NUC cessation group (p <0.0001).
– At 48 weeks post-treatment withdrawal, the PegIFN-α-2a group maintained significantly lower relapse rates (17.8% vs. 36.7%; p = 0.007).
– Clinical relapse rates, featuring ALT flares and signs of hepatic injury, were lower with PegIFN-α-2a (7.8% vs. 20.9%; p = 0.008).
– HBsAg loss rates, a marker of functional cure, were significantly higher in the PegIFN-α-2a group (21.5% vs. 9.0%; p = 0.03).
Correlation analysis revealed a positive association between baseline HBsAg quantification and likelihood of virological relapse in patients who discontinued NUC therapy without subsequent PegIFN treatment. This supports the role of HBsAg quantification as a predictive biomarker for relapse risk.
Safety and tolerability data, though not elaborated in this summary, are essential considerations given that interferon therapy is known for side effects such as flu-like symptoms and cytopenias. The study’s controlled setting and follow-up period established a clinically meaningful benefit-risk profile for the PegIFN switch strategy.
Expert Commentary
This rigorous randomized trial by Li et al. addresses a critical clinical dilemma in the management of HBeAg-negative chronic hepatitis B: how to safely discontinue long-term NUC therapy while minimizing relapse and maximizing chances for HBsAg clearance. The evidence that a 48-week course of PegIFN-α-2a post-NUC cessation drastically halves virological relapse and doubles HBsAg loss rates is compelling.
The biologic plausibility lies in PegIFN-α-2a’s immunomodulatory effects that augment host antiviral immunity and may promote cccDNA silencing, complementing the virological suppression achieved by NUCs. This integrated approach contrasts with abrupt NUC interruption, which may result in uncontrolled viral resurgence.
While promising, considerations include the need to identify ideal candidates for PegIFN transition—patients with lower baseline HBsAg levels appear most likely to benefit. Additionally, PegIFN adverse effects and contraindications must be weighed carefully in clinical decisions. Future studies could explore biomarker-guided treatment algorithms, longer-term durability of HBsAg loss, and quality of life outcomes.
Conclusion
This landmark randomized-controlled trial demonstrates that switching from long-term nucleo(s)tide analogue therapy to a defined course of pegylated interferon alpha-2a significantly reduces virological relapse and enhances functional cure rates in HBeAg-negative chronic hepatitis B patients. This pragmatic strategy addresses a major challenge in HBV management—safe treatment cessation—and facilitates progression toward ultimate viral control.
Clinicians should consider integrating PegIFN-α-2a as a consolidation therapy when contemplating NUC discontinuation, particularly in patients without cirrhosis and low HBV DNA levels. Ongoing research will refine patient selection and optimize protocols to maximize benefits while minimizing risks.
References
1. Li F, Qu L, Liu Y, et al. PegIFN alpha-2a reduces relapse in HBeAg-negative patients after nucleo(s)tide analogue cessation: A randomized-controlled trial. J Hepatol. 2025 Feb;82(2):211-221. doi: 10.1016/j.jhep.2024.07.019.
2. European Association for the Study of the Liver. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol. 2017 Aug;67(2):370-398.
3. Terrault NA, Lok ASF, McMahon BJ, et al. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 Hepatitis B Guidance. Hepatology. 2018 Apr;67(4):1560-1599.