Highlights
- PD-1/PD-L1 inhibitor resistance remains a critical challenge in oncology, limiting the long-term efficacy of immunotherapy.
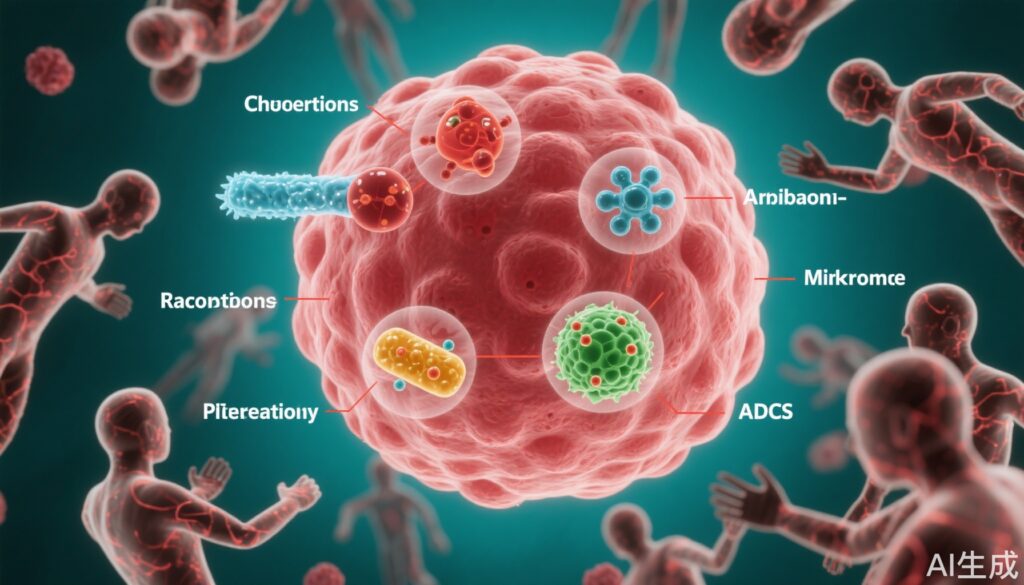
- Six combination strategies—chemotherapy, anti-angiogenic agents, radiotherapy, next-generation checkpoint inhibitors, gut microbiome modulation, and antibody-drug conjugates (ADCs)—demonstrate promise in overcoming resistance.
- Recent clinical trials, including data from the 2025 ASCO Annual Meeting, provide robust evidence supporting these approaches across multiple tumor types.
- Precision treatment selection based on patient and tumor characteristics is essential for optimizing benefit.
Background and Clinical Need
The advent of immune checkpoint inhibitors (ICIs), particularly those targeting programmed death-1 (PD-1) and its ligand PD-L1, has revolutionized the management of a spectrum of malignancies, yielding durable responses in subsets of patients. Yet, the development of primary or acquired resistance to PD-1 inhibitors presents a substantial unmet medical need. Resistance mechanisms are multifactorial, involving tumor-intrinsic genetic and epigenetic alterations, immune microenvironment modulation, and host factors—including the microbiome. With more patients receiving ICIs, strategies to overcome resistance are an urgent priority for improving survival and quality of life.
Mechanisms of Resistance to PD-1 Inhibitors
Resistance arises from complex tumor-immune interactions. Tumor cells may evade immune surveillance by downregulating antigen presentation (e.g., reducing neoantigen burden, loss of HLA expression), activating alternative oncogenic pathways (MAPK, Wnt/β-catenin), or losing tumor suppressors (PTEN). The tumor microenvironment (TME) often becomes immunosuppressive, characterized by increased regulatory T cells, myeloid-derived suppressor cells (MDSCs), and inhibitory checkpoints (e.g., TIM-3, LAG-3). Soluble factors (IDO, TGF-β, CD73) further blunt immune cell function. Emerging evidence implicates the gut microbiome in modulating ICI response, with specific commensals enhancing or impeding antitumor immunity.
Key Combination Strategies to Overcome PD-1 Resistance
1. Chemotherapy Combination
Cytotoxic chemotherapy remains integral to cancer therapy and can synergize with ICIs by inducing immunogenic cell death, releasing tumor antigens, and enhancing T cell priming. Clinical studies have demonstrated that reintroducing chemotherapy in PD-1-resistant settings can restore sensitivity. For example, cetuximab plus paclitaxel has shown efficacy and manageable toxicity in recurrent/metastatic head and neck squamous cell carcinoma post-immunotherapy. Cyclophosphamide and vinorelbine can activate stem-like CD8+ T cells, improving ICI efficacy in triple-negative breast cancer (TNBC). These regimens serve both as tumor debulking and immune adjuvant strategies.
2. Anti-Angiogenic Agents
Targeting angiogenesis disrupts the tumor’s vascular supply and reshapes the TME to favor immune cell infiltration. Agents such as lenvatinib, when combined with PD-1 inhibitors like pembrolizumab, have demonstrated impressive activity in ICI-refractory renal cell carcinoma (RCC). The KEYNOTE-146 trial reported a 55.8% objective response rate at week 24 for lenvatinib plus pembrolizumab, with manageable grade 3-4 toxicity, offering a new standard for resistant RCC.
3. Radiotherapy
Radiotherapy (RT) can potentiate immunotherapy by causing immunogenic cell death, enhancing antigen presentation, and inducing a systemic abscopal effect (tumor shrinkage outside the irradiated field). Combining RT with PD-1 inhibitors is being explored across tumor types, especially in patients with oligoprogressive or resistant disease. Early-phase data indicate that RT can convert “cold” tumors into “hot” ones, increasing immune infiltration and response rates.
4. Next-Generation Immune Checkpoint Inhibitors
Understanding alternative immune escape pathways has led to the development of novel checkpoint inhibitors targeting molecules such as CTLA-4, LAG-3, and TIGIT. The CheckMate 8HW trial, for instance, showed that nivolumab (PD-1 inhibitor) plus ipilimumab (CTLA-4 inhibitor) significantly prolonged progression-free survival (PFS) to 54.1 months in patients with MSI-H/dMMR metastatic colorectal cancer, outperforming monotherapy (39.3 months) and chemotherapy (5.9 months). This combination leverages dual mechanisms—releasing T cell inhibition and reducing regulatory T cell activity—for a synergistic antitumor effect.
5. Gut Microbiome Modulation
Several studies highlight the gut microbiome’s role in shaping ICI efficacy. Fecal microbiota transplantation (FMT) from ICI responders to resistant patients can restore sensitivity. In a clinical study of 15 PD-1-refractory melanoma patients, FMT plus anti-PD-1 therapy yielded clinical benefit in 6 patients, with notable tumor shrinkage in some. Modulating the microbiome, through diet, probiotics, or FMT, represents a promising adjunctive strategy, though safety and standardization remain under investigation.
6. Antibody-Drug Conjugates (ADCs)
ADCs combine targeted antibody specificity with cytotoxic payloads. They can eradicate tumor cells directly and elicit immunogenic cell death, enhancing the effects of ICIs. In PD-L1-positive non-small cell lung cancer (NSCLC), the ADC PF-08046054 achieved a 32% objective response rate and a median response duration of 7.8 months, with lower hematologic toxicity than standard chemotherapy. In thymic squamous carcinoma, HLX43 ADC produced a 75% response rate and 100% disease control in patients with brain metastases, demonstrating broad applicability.
Key Findings from Recent Clinical Trials
| Strategy | Tumor Type | Key Study | Notable Results | Safety |
|---|---|---|---|---|
| Chemotherapy + ICI | HNSCC, TNBC | Various | Improved ORR, T-cell activation | Manageable |
| Anti-angiogenic + ICI | RCC | KEYNOTE-146 | ORR 55.8% at 24wks | Low grade 3-4 AE |
| Radiotherapy + ICI | Multiple | Phase I/II | Increased immune infiltration | Acceptable |
| CTLA-4 + PD-1 blockade | CRC (MSI-H/dMMR) | CheckMate 8HW | PFS 54.1 mo vs 5.9 mo (chemo) | Expected immune AE |
| FMT + ICI | Melanoma | Phase I | 40% clinical benefit | Under investigation |
| ADC + ICI | NSCLC, thymic carcinoma | PF-08046054, HLX43 | ORR 32-75%, DCR 100% (CNS mets) | Low hematologic toxicity |
Expert Commentary
Current evidence strongly supports the use of rational combination therapies to address PD-1 inhibitor resistance. However, optimal sequencing, patient selection, and management of overlapping toxicities remain areas of ongoing research. Biomarker-driven approaches—including assessment of TME, TMB, and microbiome profiling—are anticipated to refine therapeutic choices further. It is also crucial to consider individual patient comorbidities, prior treatment exposure, and preferences in multidisciplinary decision-making. While the field is advancing rapidly, randomized phase III data and real-world evidence will be essential to determine the comparative benefits and risks of each strategy across different tumor types and clinical settings.
Conclusion
Resistance to PD-1 inhibitors is a significant barrier in cancer immunotherapy, but recent advances showcased at the 2025 ASCO Annual Meeting illuminate promising paths forward. Combination regimens involving chemotherapy, anti-angiogenic agents, radiotherapy, next-generation checkpoint blockade, microbiome modulation, and ADCs are reshaping the therapeutic landscape. Personalized, evidence-based integration of these modalities offers renewed hope for patients with refractory disease. Future research should focus on biomarker-guided selection, toxicity mitigation, and expanding access to innovative treatments.
References
1. Motzer RJ, et al. Lenvatinib plus pembrolizumab for advanced renal cell carcinoma. N Engl J Med. 2021;384(14):1289-1300.
2. Overman MJ, et al. Nivolumab in MSI-H/dMMR metastatic colorectal cancer: CheckMate 142. J Clin Oncol. 2018;36(8):773-779.
3. Baruch EN, et al. Fecal microbiota transplant promotes response in immunotherapy-refractory melanoma. Science. 2021;371(6529):602-609.
4. Gandhi L, et al. Pembrolizumab plus chemotherapy in metastatic NSCLC. N Engl J Med. 2018;378(22):2078-2092.
5. ASCO Annual Meeting 2025 Abstracts. (Accessed June 2025)