Highlight
- Obrixtamig (BI 764532), a DLL3/CD3 IgG-like T-cell engager, was evaluated in a phase I dose-escalation study in patients with DLL3-positive SCLC and neuroendocrine carcinomas.
- The study explored multiple dosing regimens, including step-up dosing strategies, establishing tolerability without reaching the maximum tolerated dose.
- Treatment-related cytokine release syndrome was common but mostly manageable and reversible.
- The overall response rate across all cohorts was 23%, with the highest response rate observed in large cell neuroendocrine carcinoma of the lung (70%).
- Results support further clinical investigation of obrixtamig in these difficult-to-treat DLL3-expressing tumors.
Study Background and Disease Burden
Small cell lung cancer (SCLC) and extrapulmonary neuroendocrine carcinomas (epNECs), including large cell neuroendocrine carcinoma of the lung (LCNEC-L), represent aggressive malignancies with generally poor prognosis and limited therapeutic options, particularly after multiple lines of therapy. DLL3 (delta-like ligand 3) is an inhibitory Notch ligand overexpressed in SCLC and related neuroendocrine cancers but largely absent in normal tissues, making it an attractive therapeutic target. Current treatments display limited efficacy and durable responses remain rare, thus necessitating novel strategies. Obrixtamig (BI 764532) is a bispecific T-cell engager designed to redirect CD3-positive T cells to DLL3-expressing tumor cells to trigger immune-mediated cytotoxicity, offering a new immunotherapeutic approach to treat DLL3-positive tumors.
Study Design
This first-in-human, phase I, open-label, multi-center dose-escalation trial enrolled 168 patients with previously treated DLL3-positive SCLC, epNEC, or LCNEC-L. Eligible patients had mostly undergone multiple prior lines of systemic therapy, including immune checkpoint inhibitors (51%). Obrixtamig was administered intravenously using four dosing regimens:
– Regimen A: Fixed dose once every 3 weeks
– Regimen B1: Fixed dose once weekly
– Regimen B2: Step-up dose weekly for 2 weeks, then target dose weekly
– Regimen B3: Step-up dose weekly for 3 weeks, target dose weekly for 3 weeks, followed by dosing every 3 weeks thereafter
The primary objective was to determine the maximum tolerated dose (MTD). Secondary objectives included safety profiling, pharmacokinetic evaluation, and tumor response assessment per RECIST version 1.1 criteria.
Key Findings
Among 168 patients, 72% had received at least two prior lines of anticancer therapy, reflecting a heavily pretreated population. Seven dose-limiting toxicities (DLTs) occurred during MTD evaluation—one in Regimen A and six in Regimen B2—with the MTD not reached.
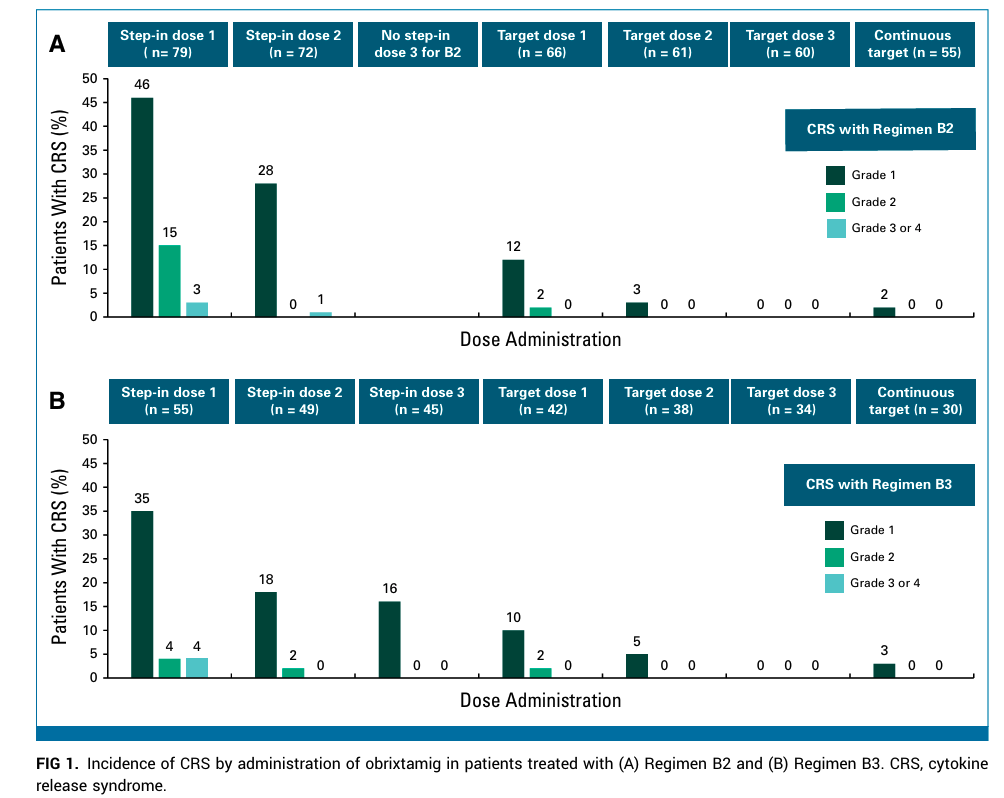
The most frequent treatment-related adverse event (AE) was cytokine release syndrome (CRS), occurring in 57% of patients across regimens. However, grade 3 or higher CRS was observed in only 3%, with symptoms typically manifesting early during treatment and reversible with appropriate management. Other adverse events were consistent with the expected safety profile of T-cell engagers.
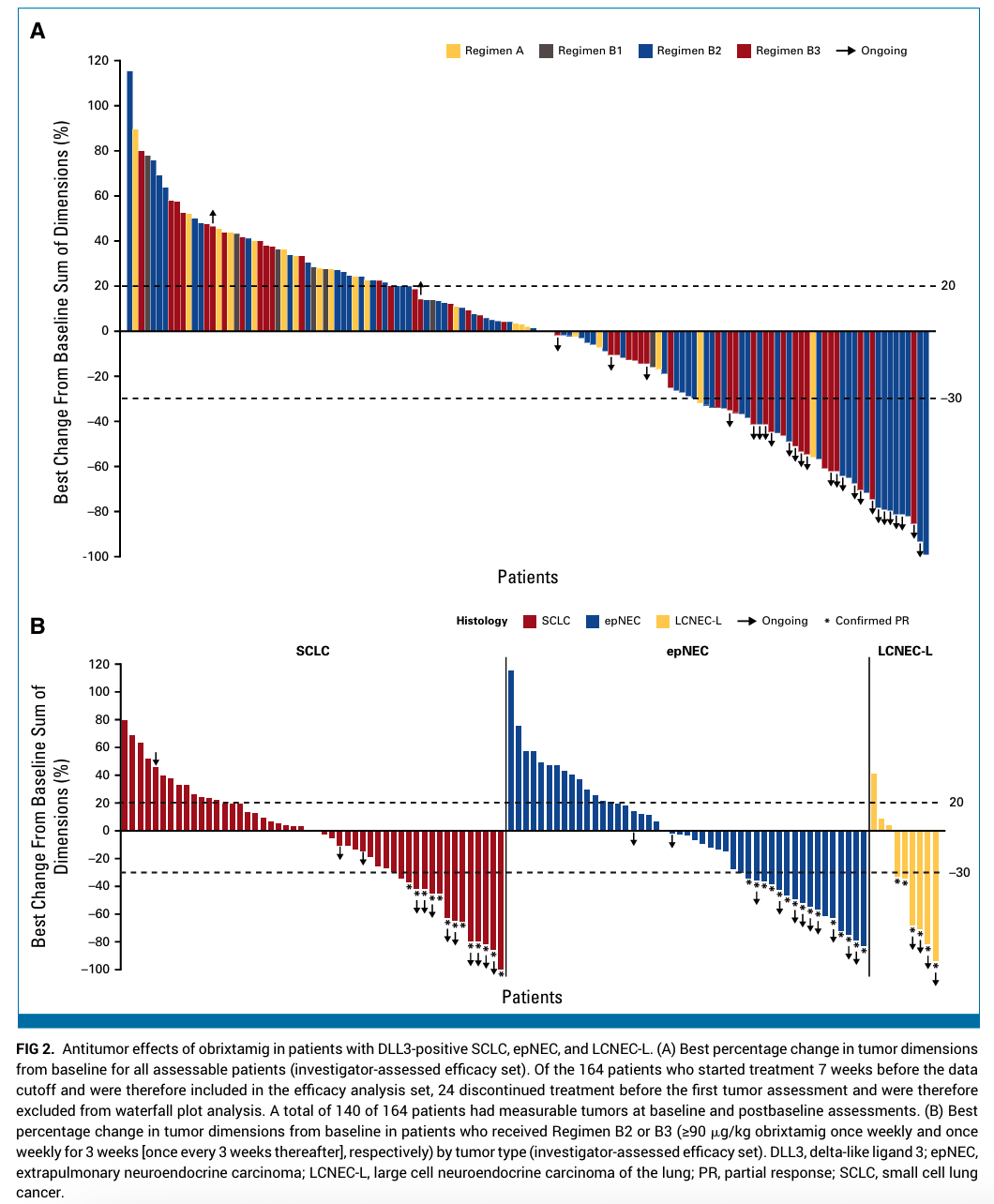
Efficacy analysis combining all regimens and tumor types demonstrated an overall response rate (ORR) of 23% (95% confidence interval [CI], 17.4% to 30.2%). The median duration of response (DoR) was 8.5 months (95% CI, 6.2 to not reached), and the 6-month DoR rate reached 70% (95% CI, 53% to 88%), indicating durable clinical benefit in responders.
Focusing on the patients receiving the minimum effective dose (≥90 μg/kg) in Regimens B2/B3, the ORR improved to 28% (95% CI, 20.7% to 35.9%), demonstrating enhanced efficacy with optimized dosing strategies.
Tumor-specific response rates with Regimens B2/B3 were:
– 21% ORR in small cell lung cancer (95% CI, 12.9% to 33.1%)
– 27% ORR in extrapulmonary neuroendocrine carcinomas (95% CI, 17.4% to 39.6%)
– 70% ORR in large cell neuroendocrine carcinoma of the lung (95% CI, 39.7% to 89.2%)
These findings indicate substantial antitumor activity, particularly in LCNEC-L, a subtype with limited prior therapeutic successes.
Pharmacokinetic assessments revealed dose-proportional increases in plasma concentrations, supporting the dosing regimens used.
Expert Commentary
The phase I results for obrixtamig represent an encouraging advance in immunotherapy targeting DLL3, an antigen notoriously challenging to exploit clinically. The manageable safety profile, predominantly mild to moderate CRS resolving early, aligns well with the known toxicities of T-cell engagers. Notably, the absence of an MTD despite observing DLTs suggests a relatively wide therapeutic window.
The durable responses in a heavily pretreated patient population—including those who previously received anti-PD-1/PD-L1 therapies—highlight obrixtamig’s potential clinical relevance as a novel treatment modality. The marked response rate in LCNEC-L is particularly notable, given this aggressive cancer’s paucity of effective therapies.
Limitations of this study include its early-phase design and lack of a comparator arm, which necessitate cautious interpretation. Further phase II/III trials are critical to confirm efficacy, define optimal dosing schedules, and explore combination approaches, potentially enhancing benefit.
Mechanistically, obrixtamig’s bispecific binding enables direct recruitment of cytotoxic T cells to tumor sites, bypassing the need for conventional antigen presentation and potentially overcoming tumor immune evasion strategies common in neuroendocrine tumors.
Conclusion
This phase I dose-escalation trial of obrixtamig demonstrates promising tolerability and preliminary efficacy in heavily pretreated patients with DLL3-positive small cell lung cancer and neuroendocrine carcinomas. Step-up dosing regimens culminating in weekly or triweekly target doses between 90 and 1080 μg/kg exhibited manageable safety and generated an overall response rate of 23%, with notable durability of response.
The particularly high response in large cell neuroendocrine carcinoma warrants further focused investigation. These results justify ongoing and future clinical trials to better define obrixtamig’s therapeutic role and optimize its integration into treatment paradigms for DLL3-expressing neuroendocrine malignancies.
References
Wermke M, Gambardella V, Kuboki Y, Felip E, Sanmamed MF, Alese OB, Sayehli CM, Arriola E, Wolf J, Villaruz LC, Bertulis J, Studeny M, Bouzaggou M, Fang X, Morgensztern D. Phase I Dose-Escalation Results for the Delta-Like Ligand 3/CD3 IgG-Like T-Cell Engager Obrixtamig (BI 764532) in Patients With Delta-Like Ligand 3+ Small Cell Lung Cancer or Neuroendocrine Carcinomas. J Clin Oncol. 2025 Sep 20;43(27):3021-3031. doi: 10.1200/JCO-25-00363 IF: 41.9 Q1 . Epub 2025 Jul 24. PMID: 40706016 IF: 41.9 Q1 ; PMCID: PMC12440289 IF: 41.9 Q1 .
Additional supporting literature:
– Rudin CM, et al. Small cell lung cancer. Nat Rev Dis Primers. 2021;7(1):3.
– Stewart CA, et al. Targeting DLL3 in pulmonary neuroendocrine tumors. Cancer Immunol Res. 2020;8(2):177-182.
– Sigma-Aldrich T-Cell Engager Mechanism Review. ImmunoOncology. 2023;9(4):254-263.