Highlight
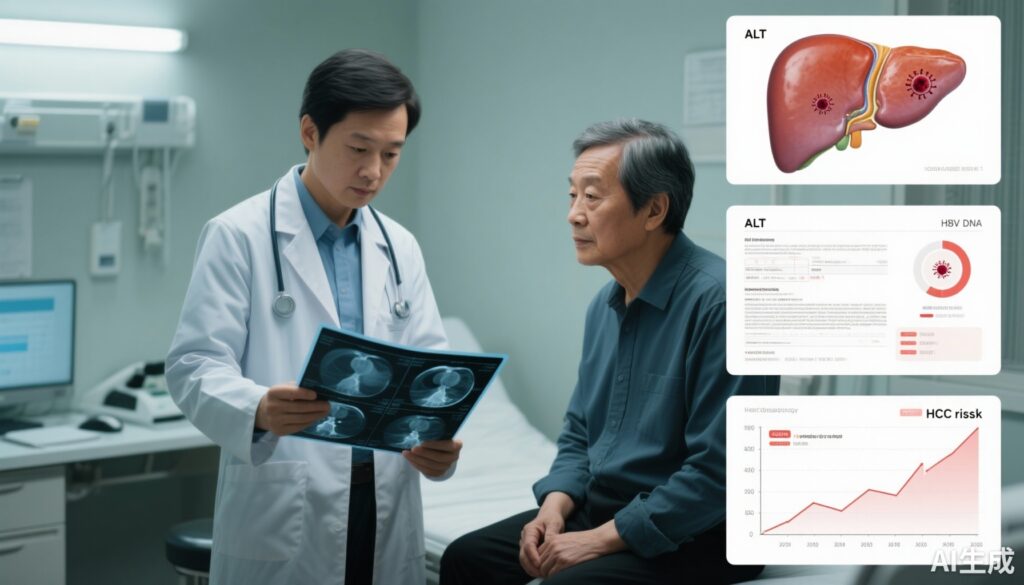
– In a multinational retrospective cohort of 1,986 patients with indeterminate chronic hepatitis B (94.2% Asian), long‑term HCC risk varied widely across prespecified indeterminate types, with 20‑year cumulative incidence ranging from 1.9% to 36.2%.
– HBeAg‑positive indeterminate types with high HBV DNA but low ALT (type 1) and with ALT 1–2×ULN (type 2) had the highest adjusted HCC risks versus a low‑risk reference (type 8): aHR 40.1 and 25.1, respectively.
– Transition from indeterminate to immune tolerant or immune active phases was associated with substantially higher cumulative HCC incidence than transition to immune inactive phase, supporting phase‑specific risk stratification and consideration of earlier antiviral therapy for high‑risk pathways.
Background
Chronic hepatitis B virus (HBV) infection is heterogeneous over time. Traditional clinical phases—immune tolerant, immune active, immune inactive and HBeAg‑positive or negative—help guide antiviral therapy and surveillance. However, many patients do not fit neatly into those categories and are labelled “indeterminate.” This indeterminate group is clinically heterogeneous and its natural history, particularly long‑term hepatocellular carcinoma (HCC) risk, has been incompletely characterized. Current treatment guidelines (AASLD, EASL) use ALT, HBV DNA, HBeAg status and fibrosis to decide treatment; uncertainty remains about which indeterminate patients should be observed versus offered antiviral therapy.
Study design
Huang and colleagues performed a retrospective multinational cohort analysis including 1,986 patients with indeterminate CHB from nine countries/regions (94.2% Asian). Patients were classified at baseline into indeterminate subtypes using permutations of three routine variables: hepatitis B e‑antigen (HBeAg) status, alanine aminotransferase (ALT) relative to upper limit of normal (ULN), and HBV DNA level. One subgroup (type 10) also incorporated histologic assessment (moderate to severe inflammation/fibrosis) despite low viral load. The primary endpoint was cumulative incidence of HCC over long‑term follow‑up; analyses compared baseline subtypes and phase transitions (i.e., how patients changed to canonical phases over time). Multivariable Cox regression adjusted for covariates assessed relative HCC hazard by subtype. Sensitivity analyses were performed using European (EASL 2017) guideline thresholds as well as AASLD 2018 criteria.
Key findings
Overall cohort and subtype distribution
Most indeterminate patients were HBeAg‑negative (84.9%) per AASLD 2018 guidance. The cohort allowed direct comparison across clinically plausible indeterminate permutations, enabling subgroup‑level risk assessment rather than treating indeterminate CHB as a single entity.
Long‑term HCC incidence by baseline subtype
Twenty‑year cumulative HCC incidence differed markedly by subtype. Using the AASLD‑based classification, the extremes were:
- Type 1 (HBeAg positive; ALT <1×ULN; HBV DNA 20,000–10^6 IU/mL): 20‑year HCC incidence 36.2%.
- Type 8 (HBeAg negative; ALT 1–2×ULN; HBV DNA <2,000 IU/mL): 20‑year HCC incidence 1.9%.
- Patients who remained indeterminate during follow‑up: 20‑year incidence 4.7%.
Phase transition and HCC risk
Risk also depended on the phase to which patients transitioned:
- Transition to immune tolerant: 15‑year cumulative HCC incidence 16.5%.
- Transition to immune active: 20‑year cumulative HCC incidence 13.7%.
- Transition to immune inactive: 20‑year cumulative HCC incidence 2.5%.
Adjusted comparisons
In multivariable models using type 8 as the referent, significantly higher HCC hazards were observed for several indeterminate types:
- Type 1 (HBeAg positive, high HBV DNA, low ALT): adjusted hazard ratio (aHR) = 40.1; p < 0.001.
- Type 2 (HBeAg positive or negative with ALT 1–2×ULN and HBV DNA ≥20,000 IU/mL): aHR = 25.1; p < 0.001.
- Type 9 (HBeAg negative, ALT >2×ULN, HBV DNA <2,000 IU/mL): aHR = 4.6; p = 0.032.
- Type 10 (HBeAg negative, ALT <1×ULN, HBV DNA <2,000 IU/mL but with moderate to severe inflammation/fibrosis on histology): aHR = 7.3; p = 0.033.
Sensitivity analyses applying 2017 EASL thresholds showed similar directional differences in HCC risk across indeterminate subtypes, supporting robustness to guideline definition variability.
Implications for surveillance and treatment
The data identify a subset of patients labelled indeterminate who have HCC risk comparable to, or higher than, groups traditionally targeted for antiviral therapy and HCC surveillance. In particular, HBeAg‑positive indeterminate types with high HBV DNA but low ALT (type 1) and those with modest ALT elevation and high HBV DNA (type 2) carried very high long‑term HCC risk. Additionally, patients with low HBV DNA but significant histologic inflammation/fibrosis (type 10) and those with high ALT despite low HBV DNA (type 9) had elevated risk compared with low‑risk referents.
Expert commentary and interpretation
These findings challenge the assumption that “indeterminate” status uniformly confers moderate or low risk. The study’s large, geographically diverse cohort and long follow‑up allow meaningful stratification. Several biologic and clinical mechanisms can explain the observations. High HBV replication in the presence of minimal ALT elevation (classic immune tolerant phenotype) can result in long‑standing viral‑host interactions that promote oncogenesis, particularly when HBeAg persists. Conversely, patients with low serum HBV DNA but significant necroinflammation or fibrosis likely have cumulative liver injury that increases HCC risk despite low current viremia.
From a guideline and clinical‑practice perspective, the data support more nuanced risk assessment in indeterminate CHB: (1) classify indeterminate patients by HBeAg, HBV DNA, ALT and fibrosis/inflammation status; (2) recognize that some indeterminate types (particularly types 1 and 2) have HCC risks that would justify considering antiviral therapy earlier than currently recommended; and (3) intensify HCC surveillance in high‑risk indeterminate subgroups.
Study strengths
Strengths include cohort size, multinational representation, long follow‑up (up to 20 years), prespecified subtype definitions aligned with real‑world guideline thresholds, and multivariable adjustment and sensitivity analyses that preserved findings across classification approaches.
Limitations
Key limitations must temper conclusions. The study is retrospective and observational, so unmeasured confounding and selection bias are possible. The cohort was predominantly Asian (94.2%), which may limit generalizability to other ethnic groups with different HBV genotypes and HCC baseline risks. Definitions of ALT ULN varied historically and across centers; single timepoint classification may misclassify fluctuating disease activity. Treatment exposure during follow‑up (timing, adherence, drug potency) could modify lagging HCC risk and needs careful interpretation. Finally, although the associations are strong for some subtypes, causal inference that antiviral therapy will reduce HCC in these specific indeterminate subgroups requires prospective evaluation.
Clinical implications and recommendations
For practicing clinicians managing patients with indeterminate CHB, practical takeaways are:
- Do not treat “indeterminate” as a homogeneous low‑risk label. Assess HBeAg, quantitative HBV DNA, ALT trends and fibrosis (by noninvasive tests or histology) to stratify risk.
- Patients who meet the high‑risk indeterminate profiles (especially type 1 and type 2 in this study) merit a low threshold for antiviral therapy discussion and might benefit from enrolment in enhanced HCC surveillance programmes.
- Those with low HBV DNA but significant fibrosis or elevated ALT (types 9 and 10) should be evaluated carefully; histologic or elastographic fibrosis assessment can change management decisions.
- Shared decision‑making should incorporate comorbidities, age, family history of HCC, and patient preferences alongside the new risk data.
Research and policy implications
These results can inform guideline panels considering expanded treatment criteria. Prospective studies and randomized trials comparing early antiviral therapy versus observation in high‑risk indeterminate types are needed to determine whether treating before classic immune active criteria are met reduces HCC incidence, improves mortality, and is cost‑effective. Health systems should consider how to implement refined risk stratification algorithms and ensure access to fibrosis assessment and HCC surveillance for patients reclassified as higher risk.
Conclusion
Huang et al. provide compelling evidence that indeterminate CHB is heterogeneous with respect to long‑term HCC risk. Specific indeterminate subtypes—most notably HBeAg‑positive with high HBV DNA and low ALT—have markedly elevated HCC incidence and adjusted hazard compared with low‑risk indeterminate types. These data support individualized risk stratification within the indeterminate category and argue for consideration of earlier antiviral therapy and targeted surveillance in higher‑risk subgroups. Prospective trials and guideline deliberation are warranted to translate these observational findings into practice change.
Selected references
Huang R, Trinh HN, Yasuda S, Chau A, Maeda M, Do AT, Huang DQ, et al. Differential HCC risk among HBV indeterminate types at baseline and by phase transition. Gut. 2025 Oct 8;74(11):1873–1882. doi:10.1136/gutjnl-2025-335033. PMID: 40467102.
Terrault NA, Lok ASF, McMahon BJ, Chang KM, Hwang JP, Jonas MM, Brown RS Jr, et al. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology. 2018;67(4):1560–1599.
European Association for the Study of the Liver. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol. 2017;67(2):370–398.
Funding and trial registration: See primary manuscript for detailed funding sources and cohort registration details.