Highlights
- Perioperative pembrolizumab added to standard surgery and adjuvant therapy significantly improves 3-year event-free survival in locally advanced head and neck squamous-cell carcinoma (HNSCC).
- Benefit observed across subgroups based on PD-L1 expression levels (CPS ≥10 and CPS ≥1) as well as the overall patient population.
- Neoadjuvant pembrolizumab does not reduce surgical completion rates, emphasizing its feasibility in the perioperative setting.
- Safety profile consistent with known pembrolizumab toxicities, with immune-mediated adverse events manageable and no unexpected safety signals.
Background
Head and neck squamous-cell carcinoma (HNSCC) represents a major global health burden due to its aggressive nature and propensity for recurrence despite standard multimodal therapy, including surgery and radiotherapy with or without chemotherapy. Although immune checkpoint inhibitors targeting the programmed death 1 (PD-1) axis have revolutionized recurrent/metastatic HNSCC management, their role as perioperative therapy in locally advanced disease has been unclear.
Perioperative administration of immune checkpoint inhibitors, including neoadjuvant and adjuvant approaches, aims to enhance antitumor immune responses, eradicate micro-metastatic disease, and improve long-term outcomes. The combined positive score (CPS) of PD-L1 expression serves as a predictive biomarker for immunotherapy response in HNSCC, with higher levels correlating with increased benefit.
Key Content
Study Design and Patient Population
The KEYNOTE-689 trial (NCT03765918) is a phase 3, open-label, randomized controlled study that enrolled 714 participants with locally advanced HNSCC. Patients were randomized 1:1 to receive either standard care alone—surgery followed by adjuvant radiotherapy with or without cisplatin—or combined with pembrolizumab dosing (two cycles neoadjuvant pembrolizumab plus fifteen cycles adjuvant pembrolizumab at 200 mg every three weeks).
Patients were stratified based on PD-L1 expression, measured by the combined positive score (CPS), with analyses conducted in three cohorts: CPS≥10, CPS≥1, and the entire intention-to-treat population.
Primary Efficacy Outcomes
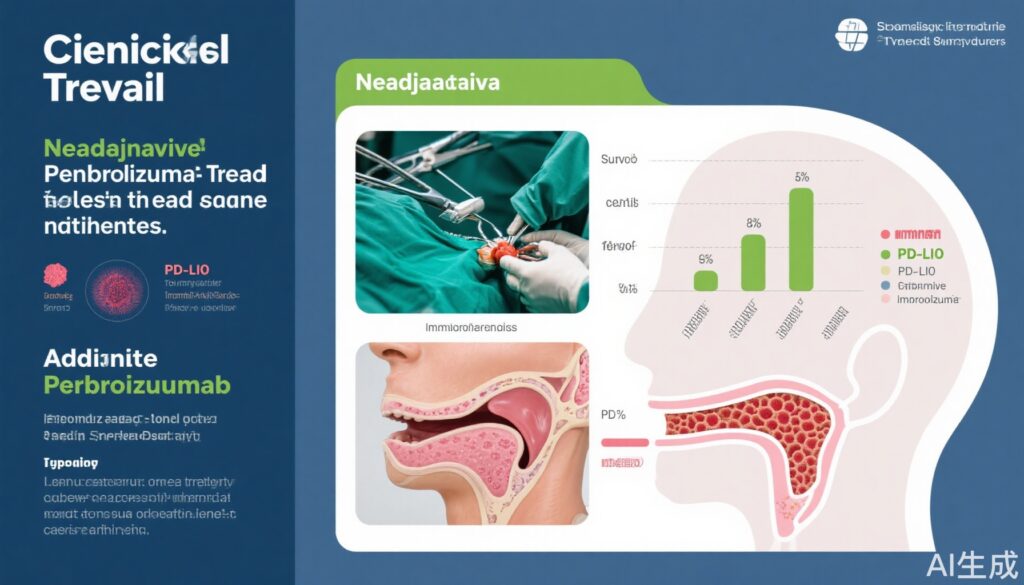
At a median follow-up of 38.3 months, pembrolizumab addition achieved statistically significant improvements in event-free survival (EFS):
- CPS-10 population: 36-month EFS was 59.8% versus 45.9% in control (HR 0.66; 95% CI, 0.49-0.88; P=0.004).
- CPS-1 population: 58.2% versus 44.9% in control (HR 0.70; 95% CI, 0.55-0.89; P=0.003).
- Overall population: 57.6% versus 46.4% in control (HR 0.73; 95% CI, 0.58-0.92; P=0.008).
These results demonstrate a consistent benefit across biomarker strata, indicating that pembrolizumab enhances long-term disease control beyond standard surgery and adjuvant radiation/chemotherapy.
Surgical Feasibility and Safety Profile
Surgical completion rates were comparable between groups (~88%), confirming that neoadjuvant pembrolizumab does not delay or compromise surgery eligibility. Treatment-related grade 3 or higher adverse events occurred with similar frequency in the pembrolizumab (44.6%) and control (42.9%) groups, with treatment-related deaths rare (1.1% vs. 0.3%). Importantly, immune-mediated toxicities of grade 3 or higher occurred in 10.0% of patients receiving pembrolizumab, consistent with known immune checkpoint inhibitor profiles.
Mechanistic and Translational Insights
Pembrolizumab, a monoclonal antibody targeting PD-1, reinvigorates exhausted T-cell responses allowing enhanced antitumor immunity. The neoadjuvant administration likely primes systemic immune responses when tumor antigen burden is intact, potentially leading to immune memory that reduces recurrence risk. The adjuvant continuation maintains immune surveillance during a critical window of micrometastatic disease eradication.
The use of CPS as a predictive biomarker is underscored by improved outcomes especially in CPS≥10 tumors, linking PD-L1 expression levels to potentiated immunotherapeutic efficacy.
Expert Commentary
KEYNOTE-689 represents a landmark study establishing the clinical benefit of perioperative pembrolizumab in locally advanced HNSCC. Its robust design and comprehensive biomarker-based analysis provide high-level evidence supporting this immunotherapy incorporation into curative-intent regimens.
Guidance from oncology societies will likely evolve to recommend neoadjuvant and adjuvant checkpoint blockade particularly for PD-L1 positive patients. However, continued vigilance regarding immune-related adverse events and long-term outcomes is warranted.
Some limitations include the open-label design and the need for long-term overall survival data. Comparative effectiveness versus alternative emerging immunotherapeutic or targeted approaches requires further research.
Conclusion
Perioperative pembrolizumab added to standard surgical and adjuvant therapy significantly improves event-free survival in patients with locally advanced HNSCC, offering a new frontline immunotherapeutic strategy. This approach is feasible, safe, and effective across PD-L1 expression levels with manageable toxicity, representing a tangible advance in multimodal head and neck cancer treatment.
Future directions include optimizing patient selection, integrating molecular biomarkers, and combining immunotherapy with novel agents to further enhance outcomes.
References
- Uppaluri R, Haddad RI, Tao Y, Le Tourneau C, Lee NY, Westra W, et al. Neoadjuvant and Adjuvant Pembrolizumab in Locally Advanced Head and Neck Cancer. N Engl J Med. 2025 Jul 3;393(1):37-50. doi:10.1056/NEJMoa2415434. PMID: 40532178.
- Grau C, et al. Immune checkpoint blockade in head and neck cancer – rationale and future directions. Oral Oncol. 2023;141:105574. doi:10.1016/j.oraloncology.2023.105574.
- Ferris RL, et al. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med. 2016;375(19):1856-1867. doi:10.1056/NEJMoa1602252.
- Clifford G, et al. PD-L1 as a biomarker in head and neck squamous cell carcinoma: current perspectives and future potential. Front Oncol. 2024;14:1002957. doi:10.3389/fonc.2024.1002957.