Introduction
Biliary tract cancers (BTC), encompassing intrahepatic and extrahepatic cholangiocarcinoma as well as gallbladder cancer, represent a heterogeneous group of malignancies with poor prognosis due to late-stage diagnosis and aggressive tumor biology. Systemic chemotherapy remains the mainstay for unresectable or metastatic BTC. While gemcitabine plus cisplatin (GemCis) has been the standard first-line treatment, the addition of immunotherapy agents such as durvalumab or pembrolizumab has recently demonstrated survival benefits. Despite these advances, disease progression after first-line therapy is common, underscoring the need for effective subsequent treatments. Current guidelines favor FOLFOX as second-line therapy, particularly in patients without actionable mutations. However, alternative regimens remain an area of active investigation.
Liposomal irinotecan (nal-IRI) combined with fluorouracil and leucovorin (5-FU/LV) is approved in advanced pancreatic cancer after gemcitabine failure. Two phase 2 trials—the Korean NIFTY and German NALIRICC—evaluated nal-IRI plus 5-FU/LV in gemcitabine-pretreated BTC patients, yielding conflicting results. This incongruity prompted an individual patient-level pooled analysis to assess efficacy and safety comprehensively across ethnic populations.
Study Design and Methods
This pooled analysis incorporated intention-to-treat data from two multicenter randomized open-label trials, NIFTY (n=178) and NALIRICC (n=100), enrolling patients with advanced BTC who progressed following gemcitabine-based regimens. Participants received either nal-IRI (70 mg/m2 intravenous) plus 5-FU/LV or 5-FU/LV alone every two weeks. Radiological assessments were performed bi-monthly per RECIST v1.1 criteria. Primary endpoint was progression-free survival (PFS) as determined by investigators; secondary endpoints included overall survival (OS), objective response rates (ORR), and safety. Data on patient demographics, tumor characteristics, prior treatments, and adverse events (AEs) were pooled for analysis. Statistical comparisons employed stratified Cox proportional hazards models, Kaplan-Meier survival estimates, and subgroup analyses accounting for trial effect and patient heterogeneity.
Key Findings
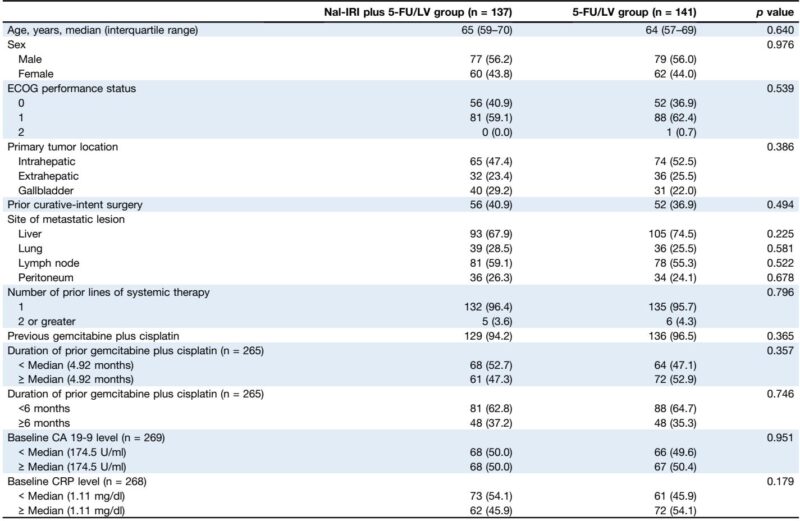
A total of 278 patients were analyzed (nal-IRI plus 5-FU/LV, n=137; 5-FU/LV, n=141). Baseline characteristics—including age, sex, ECOG performance status, and prior treatment exposure—were balanced. The nal-IRI combination significantly improved median PFS to 3.6 months (95% CI: 2.7-4.4) versus 1.8 months (95% CI: 1.5-2.6) with 5-FU/LV alone (hazard ratio [HR] 0.65; p<0.001). Median OS showed improvement (8.1 vs. 6.1 months) with borderline significance (HR 0.77; p=0.051). Notably, ORR was markedly higher with nal-IRI plus 5-FU/LV (17.5%) compared to 2.8% with 5-FU/LV (p<0.001). Subgroup analyses confirmed consistent PFS benefits across most demographic and clinical categories.
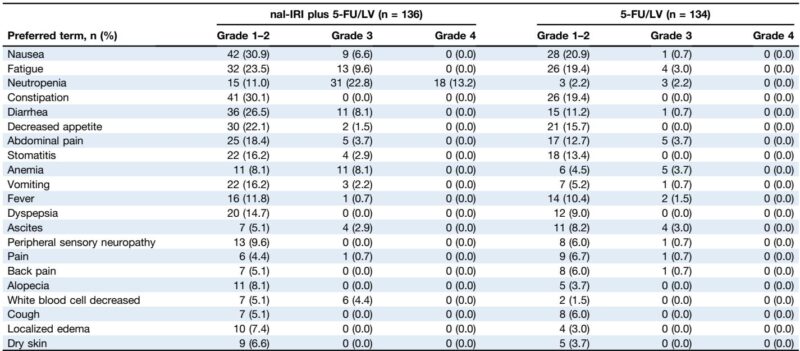
Post-study irinotecan-containing therapies were more frequently administered in the 5-FU/LV arm (15.3%) than the nal-IRI arm (2.9%), potentially impacting OS outcomes. Safety profiling demonstrated that nal-IRI addition elevated grade 3-4 toxicities, primarily neutropenia, fatigue, and diarrhea. Ethnic disparities were evident: German patients exhibited more gastrointestinal toxicities (nausea, vomiting, diarrhea), while Korean patients experienced higher rates of neutropenia. Correspondingly, treatment discontinuation without progression was higher in Germans (31.3%) versus Koreans (8.0%). Dose reduction was more common among Koreans, correlating with better treatment adherence.
Expert Commentary
This pooled analysis clarifies prior discordant trial results by leveraging a larger, ethnically diverse cohort. The addition of nal-IRI to 5-FU/LV confers clear progression-free survival and response benefits in BTC patients after gemcitabine failure, supporting its incorporation as a subsequent-line option, especially in East Asian populations. The lack of a definitive OS advantage may relate to differential subsequent treatments and toxicity-induced discontinuations, particularly in the German cohort.
Ethnic variations in toxicity, underpinned by pharmacokinetic differences, highlight the necessity for personalized dosing and vigilant toxicity management. Gastrointestinal adverse events chiefly affected European patients, potentially causing premature cessation and depriving them of maximal therapeutic benefit. Conversely, higher hematologic toxicity among Koreans did not lead to frequent discontinuation, likely reflecting proactive dose modifications.
Given FOLFOX’s established second-line role, nal-IRI plus 5-FU/LV may serve as an alternative for patients intolerant to oxaliplatin or those without identifiable molecular targets. The liposomal formulation enhances irinotecan delivery and intratumoral SN-38 levels, offering a pharmacological rationale for improved efficacy.
Limitations include the unplanned nature of the pooled analysis and heterogeneity in prior treatments. Nonetheless, this study exemplifies fruitful international collaboration in a rare malignancy and emphasizes ethnic considerations in chemotherapy administration.
Conclusion
Liposomal irinotecan combined with fluorouracil and leucovorin significantly extends progression-free survival and improves response rates in advanced biliary tract cancer patients refractory to gemcitabine-based therapy. Ethnic differences in adverse event profiles necessitate tailored management to optimize outcomes. While overall survival improvement awaits confirmation, nal-IRI plus 5-FU/LV represents a valuable second-line therapeutic option, expanding existing arsenals for this challenging disease. Further randomized studies are warranted to consolidate these findings and refine patient selection strategies.
References
Yoo C, Saborowski A, Hyung J, Wenzel P, Kim I, Wege H, Kim KP, Folprecht G, Ryoo BY, Schütt P, Cheon J, Götze T, Ryu H, Lee JS, Vogel A. Liposomal irinotecan for previously treated patients with biliary tract cancer: A pooled analysis of NIFTY and NALIRICC trials. J Hepatol. 2025 Oct;83(4):909-916. doi: 10.1016/j.jhep.2025.03.013 IF: 33.0 Q1 . Epub 2025 Mar 25. PMID: 40147791 IF: 33.0 Q1 .
