Highlights
- Inhaled isoflurane matched intravenous midazolam for sedation efficacy in critically ill, mechanically ventilated children (ages 3-17).
- Isoflurane use resulted in significantly lower opioid requirements and shorter time to extubation compared to midazolam.
- The safety profile of isoflurane was acceptable, with no treatment-related deaths in the multicenter trial.
- Supported by trial evidence and EMA guidance, inhaled isoflurane is now a viable sedative option in pediatric intensive care.
Clinical Background and Disease Burden
Sedation is foundational in the management of critically ill children requiring mechanical ventilation in pediatric intensive care units (PICUs). Achieving adequate sedation minimizes pain, anxiety, accidental extubation, and ventilator asynchrony, ultimately improving patient outcomes. Traditionally, intravenous benzodiazepines like midazolam have been favored for their rapid onset and titratability. However, concerns have emerged regarding tolerance, withdrawal, paradoxical agitation, and potential neurotoxicity, especially in prolonged use. Additionally, the opioid-sparing potential of alternative sedative regimens is highly desirable given the side effects and dependency risks associated with opioids.
Inhaled anesthetics, such as isoflurane, offer rapid onset and offset, minimal accumulation, and predictable pharmacokinetics, making them attractive for short- to medium-term ICU sedation. Despite their routine use in operative anesthesia, their role in ICU sedation, particularly in children, has remained underexplored due to technical and safety concerns.
Research Methodology
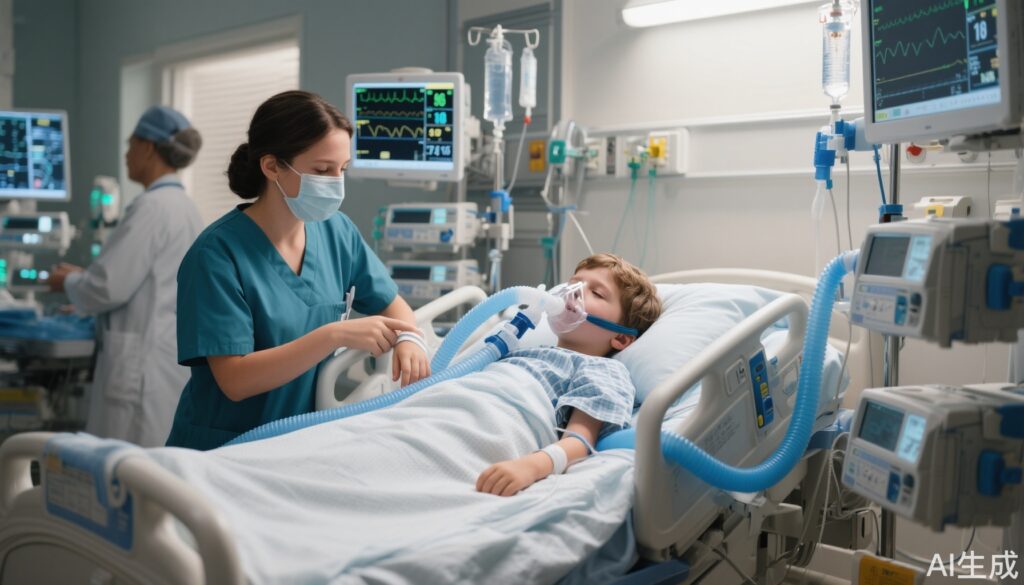
The IsoCOMFORT trial (Miatello J et al., Lancet Respir Med. 2025) was a multicenter, randomized, active-control, assessor-masked, non-inferiority phase 3 study conducted across multiple European PICUs. Ninety-two critically ill children aged 3 to 17 years, all requiring invasive mechanical ventilation and sedation for at least 12 hours, were enrolled. Participants were randomly assigned in a 2:1 ratio to receive either inhaled isoflurane (n = 59) or intravenous midazolam (n = 33). Baseline characteristics were well-matched (mean age: isoflurane 8.1 years, midazolam 7 years; approximately 60% boys in both groups).
Sedation targets were individualized using the validated COMFORT Behavior Scale, classifying patients into light, moderate, or deep sedation categories. Sedative dosing was titrated over a 48 ± 6 hour treatment window, and depth of sedation was assessed every two hours. The primary endpoint was the proportion of time within the prescribed COMFORT Behavior Scale target range without the need for rescue sedation.
Key Findings
The study demonstrated that isoflurane maintained adequate sedation within the target range for a mean of 68.94% of the monitored time, compared to 62.37% in the midazolam group. The mean difference (6.57 percentage points; 95% CI, -8.99 to 22.13) established the noninferiority of isoflurane, as the lower confidence bound did not cross the prespecified margin.
Isoflurane use also resulted in several clinically meaningful secondary benefits:
- Reduced opioid requirements: Children in the isoflurane arm required a median opioid dose of 2.1 μg/kg/h versus 4.6 μg/kg/h in the midazolam group (P = .0004), highlighting a robust opioid-sparing effect.
- Faster extubation: Median time to extubation post-sedation was shorter with isoflurane (0.75 hours) compared to midazolam (1.09 hours).
- Safety: Severe hypotension occurred in one child in each group; three children discontinued isoflurane due to adverse events, but there were no treatment-related deaths.
These data suggest that isoflurane not only matches midazolam in efficacy but also offers tangible advantages in opioid stewardship and ICU throughput.
Mechanistic Insights and Biological Plausibility
Isoflurane, a volatile anesthetic, exerts its sedative effects primarily through potentiation of γ-aminobutyric acid (GABA) type A receptors and inhibition of excitatory neurotransmission. Its rapid onset and clearance via the respiratory tract (rather than hepatic or renal metabolism) reduce the risk of drug accumulation, especially during prolonged sedation. The observed opioid-sparing effect may be attributed to isoflurane’s intrinsic analgesic properties and superior control of agitation and distress.
Expert Commentary
The study authors, supported by regulatory endorsement from the European Medicines Agency (EMA), recommend that inhaled isoflurane be considered a therapeutic option for pediatric ICU sedation. This aligns with a growing body of opinion advocating broader use of inhaled sedation techniques, particularly where intravenous options may pose additional risks. However, routine adoption will require education, technical support for vaporizer systems, and protocols to ensure occupational safety.
Controversies and Limitations
While the IsoCOMFORT trial provides important evidence, several limitations should be acknowledged:
- Sample size: The study enrolled 92 participants—a modest cohort for a multicenter trial, potentially limiting statistical power for infrequent adverse events.
- Generalizability: The trial was conducted across European PICUs, and findings may not fully extrapolate to regions with differing ICU resources or sedation practices.
- Technical requirements: Implementation of inhaled sedation necessitates specialized equipment for gas delivery and scavenging, which may not be universally available.
- Long-term outcomes: The study did not evaluate neurodevelopmental outcomes, which remain a concern with both benzodiazepines and volatile anesthetics.
Conclusion
The IsoCOMFORT trial robustly supports inhaled isoflurane as a noninferior alternative to intravenous midazolam for sedation in mechanically ventilated children, with the added benefits of reduced opioid exposure and expedited extubation. Its safety profile was acceptable in the study context. Given ongoing concerns with intravenous benzodiazepines and increasing regulatory support, inhaled isoflurane should be considered in pediatric ICU sedation protocols, contingent on local expertise and resources. Future research is warranted to explore long-term neurocognitive effects and optimize implementation.
References
1. Miatello J, Palacios-Cuesta A, Radell P, Oberthuer A, Playfor S, Amores-Hernández I, et al.; IsoCOMFORT Study Group. Inhaled isoflurane for sedation of mechanically ventilated children in intensive care (IsoCOMFORT): a multicentre, randomised, active-control, assessor-masked, non-inferiority phase 3 trial. Lancet Respir Med. 2025 Jul 15:S2213-2600(25)00203-6. doi: 10.1016/S2213-2600(25)00203-6. Epub ahead of print. PMID: 40680761.
2. Playfor SD, Smith RL, Davis PJ. Sedation and analgesia in the critically ill child. Paediatr Anaesth. 2006;16(5):448-56.
3. Davidson AJ, Disma N, de Graaff JC, et al. Neurodevelopmental outcome at 2 years of age after general anaesthesia and awake-regional anaesthesia in infancy (GAS): an international multicentre, randomised controlled trial. Lancet. 2016;387(10015):239-250.