Depression is a pervasive psychiatric disorder characterized by prolonged depressed mood, anhedonia, and associated cognitive and somatic symptoms. Globally, major depressive disorder (MDD) affects up to 21% of populations, with significant functional impairment and healthcare costs. Conventional treatment includes psychological therapies and pharmacotherapies—primarily antidepressants. However, challenges such as treatment accessibility, side effects, and patient preference have led many individuals to self-manage depressive symptoms using over-the-counter (OTC) herbal medical products (HMPs) and dietary supplements. Despite widespread use, a comprehensive understanding of the research landscape surrounding these OTC products for adult depression is lacking. Addressing this gap enables clinicians and researchers to identify promising interventions, optimize clinical recommendations, and direct future research efforts efficiently.
Study Design and Methodology
A rigorous scoping review followed the Joanna Briggs Institute and PRISMA-ScR guidelines, surveying records from MEDLINE, Embase, PsycINFO, AMED, and CENTRAL databases from inception through December 2022. Inclusion criteria centred on randomized controlled trials (RCTs) evaluating single-ingredient OTC products, including HMPs, dietary supplements, and single chemical medicines, administered orally to adults aged 18-60 years with depressive symptoms or diagnoses. Studies involving comorbid conditions were eligible; however, those focusing on other primary psychiatric disorders were excluded. Outcomes included validated depression rating scales or remission. Both monotherapy and adjunctive interventions were considered. Data extraction encompassed trial characteristics, interventions, comparators, outcomes, and safety reporting. Effectiveness outcomes were synthesized via vote counting due to the heterogeneity in products and trials.
Key Findings
Out of 23,933 identified records, 209 trials were included (median sample size: 70). Evidence evaluation revealed that research is heavily concentrated on five OTC products: omega-3 fatty acids (39 trials), St John’s Wort (38 trials), saffron (18 trials), probiotics (18 trials), and vitamin D (14 trials). These products demonstrated varied but often favorable effects on depressive symptoms compared to placebo or standard antidepressant treatment. Omega-3 trials showed mixed outcomes, with a trend towards benefit in EPA-predominant formulations. St John’s Wort consistently exhibited efficacy comparable or superior to prescription antidepressants with favorable tolerability. Saffron demonstrated potential as a monotherapy and adjunct, while probiotics and vitamin D showed emerging but less conclusive benefits.
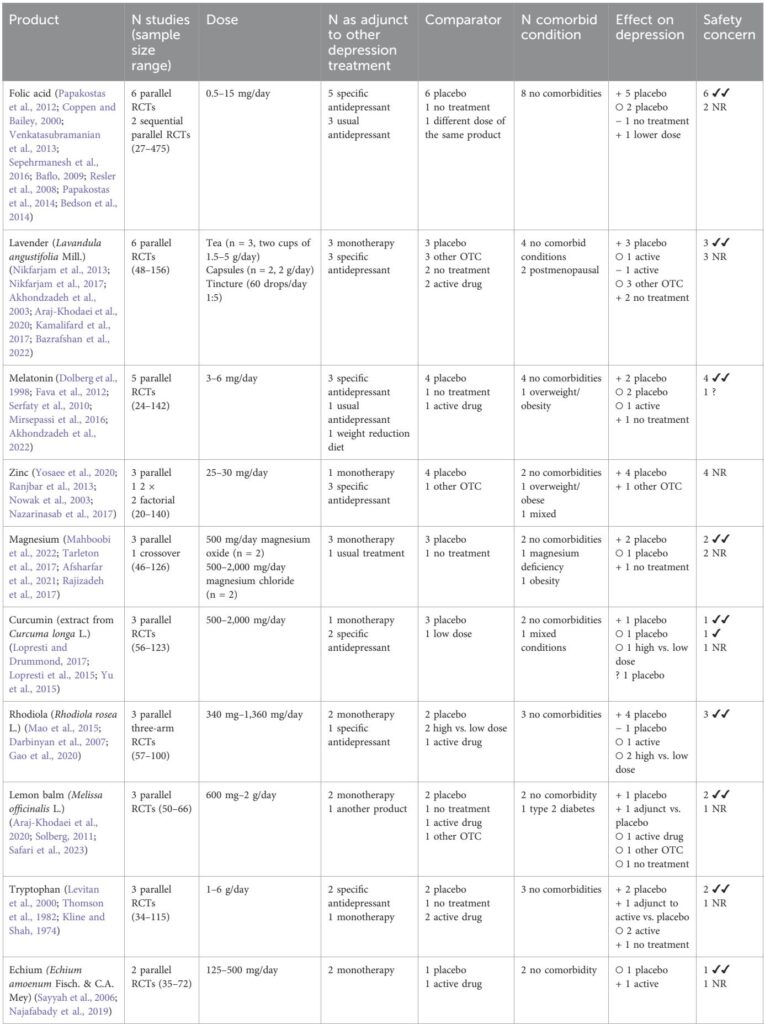
Beyond these prominent modalities, eighteen products had emerging evidence supported by 2 to 9 trials. Among these, folic acid, lavender (Lavandula angustifolia), zinc, tryptophan, Rhodiola rosea, and lemon balm showed promising antidepressant effects. Herbal teas, a common traditional preparation, were underrepresented in research, with trials predominantly using capsule formulations. A substantial number (41 products) were evaluated only once, including single ingredients such as rosemary, green tea, and Nigella sativa, some showing positive preliminary results.
Regarding safety, most trials reporting adverse events indicated good tolerability of OTC products, with only a few reporting mild differences. One omega-3 trial reported increased serious adverse events, though causality was unclear. Approximately 31% of studies inadequately reported safety data, underscoring the need for more rigorous adverse event documentation.
Economic data were sparse, with a single trial of folic acid showing no cost-effectiveness advantage over placebo from a national health system perspective.
Current ongoing trials primarily focus on probiotics, vitamin D, and omega-3s, with fewer evaluating the promising but less-studied OTC products noted above. Notably, no homoeopathic products were identified among depression trials.
The research also highlighted limited investigation of OTC products as adjuncts to psychological therapies—an area requiring attention given therapy’s first-line status in guidelines and real-world use patterns.
Expert Commentary and Context
Meta-analyses supporting these findings confirm St John’s Wort’s efficacy and safety compared to SSRIs, saffron’s antidepressant properties, and the potential antidepressant benefits of omega-3 fatty acids, especially EPA subtypes. Probiotic interventions have shown promise in modulating the gut–brain axis, which may contribute to mood improvements. Vitamin D’s role remains less definitive, with mixed results likely influenced by baseline deficiency status.
Traditional and mechanistic research shows that promising OTC products, such as lavender, chamomile, lemon balm, and Rhodiola, possess anti-inflammatory, antioxidative, neuroprotective, and neurotransmitter-modulating properties, aligning with known depression etiologies. These phytochemicals influence hypothalamic–pituitary–adrenal axis regulation, brain-derived neurotrophic factor (BDNF) expression, and overall neurogenesis.
The review reveals a discrepancy between commonly used medicinal herbal teas and the relative paucity of clinical trials assessing them. Given their cultural acceptance, availability, and cost-efficiency, herbal teas merit further clinical trials.
Limitations of the evidence include substantial heterogeneity in dosing, preparations, and study design, small sample sizes prone to overestimation of benefits, and a significant proportion of trials with poor adverse event reporting. Screening constraints and incomplete database search terms may have excluded some relevant studies.
Conclusion
This comprehensive scoping review delineates the current research landscape of OTC herbal and dietary supplements for adult depression, highlighting a concentrated evidence base for omega-3 fatty acids, St John’s Wort, saffron, probiotics, and vitamin D. It identifies several promising but under-researched products such as folic acid, lavender, zinc, tryptophan, Rhodiola, and lemon balm worthy of rigorous clinical evaluation. Adverse event reporting, dose standardization, and evaluation of combination therapy with psychological interventions emerge as critical areas for future research. Clinicians should consider the existing evidence when discussing OTC options with patients, balancing efficacy, safety, and individual preferences. Updating clinical guidelines to reflect robust OTC product evidence may improve integrative depression care.
Reference
Frost R, Zamri A, Mathew S, Salame A, Bhanu C, Bhamra SK, Bazo-Alvarez JC, Heinrich M, Walters K. Understanding the research landscape of over-the-counter herbal products, dietary supplements, and medications evaluated for depressive symptoms in adults: a scoping review. Front Pharmacol. 2025 Jul 15;16:1609605. doi: 10.3389/fphar.2025.1609605 IF: 4.8 Q1 . PMID: 40735481 IF: 4.8 Q1 ; PMCID: PMC12303899 IF: 4.8 Q1 .