Highlights
– Nurse-led community screening accelerates MASLD risk assessment and reduces patient anxiety.
– General practitioners and patients report high acceptability and trust in nurse-delivered models.
– Adoption challenges include funding, prioritization in complex cases, and lifestyle intervention support.
Study Background and Disease Burden
Metabolic dysfunction-associated steatotic liver disease (MASLD), previously known as non-alcoholic fatty liver disease (NAFLD), is rapidly becoming one of the most prevalent chronic liver conditions worldwide, associated with obesity, type 2 diabetes, and metabolic syndrome. The clinical burden is substantial, with rising rates of advanced liver disease, cardiovascular complications, and significant impact on health systems. Current care models depend heavily on specialist input for risk stratification and management, overwhelming liver clinics and resulting in delays and inefficiencies. There is a pressing need for scalable, accessible models that enable earlier identification and management of high-risk patients in the community.
Study Design
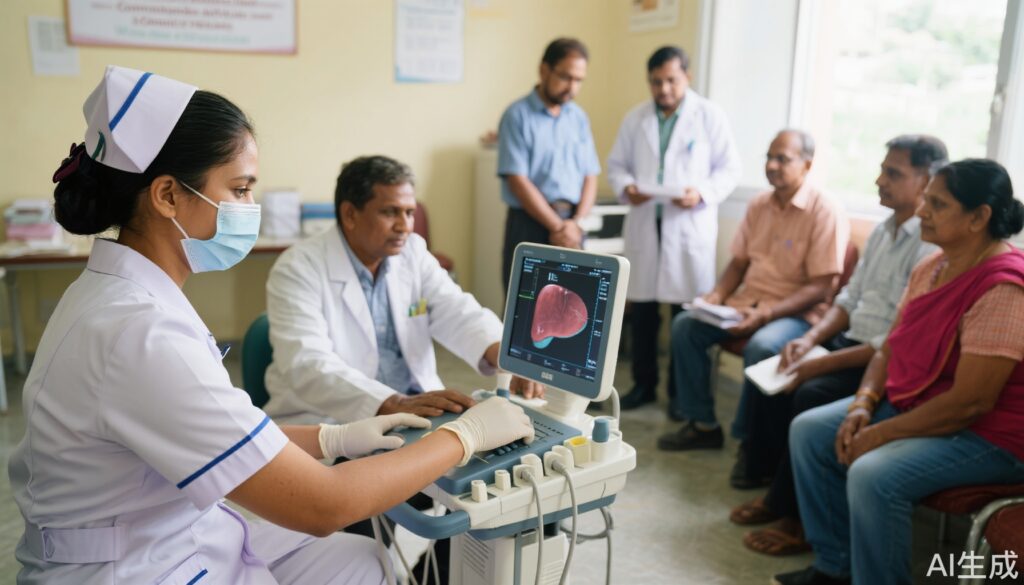
The LOCATE-NAFLD trial (LOCal Assessment and Triage Evaluation of Non-Alcoholic Fatty Liver Disease) was a randomized, mixed-methods evaluation comparing usual specialist-driven care with a nurse-delivered, community-based liver risk assessment program. The trial included patients with suspected MASLD referred from primary care across multiple Australian health services. The intervention arm received nurse-led screening and risk assessment, while the control arm followed standard referrals to specialist clinics. Outcomes were assessed using both quantitative trial data and qualitative framework analysis (semi-structured interviews), focusing on acceptability (Acceptability Framework) and implementation metrics (RE-AIM framework: Reach, Effectiveness, Adoption, Implementation, Maintenance).
Key Findings
The trial demonstrated several important outcomes:
1. Acceptability and Reach: Patients and general practitioners (GPs) reported high acceptability for the nurse-led model. The intervention achieved appropriate reach, with timely identification of high-risk individuals across participating health services.
2. Speed and Patient Experience: Nurse-delivered screening significantly reduced waiting times from referral to assessment compared to usual care. Patients in the intervention group reported lower anxiety and stress, attributing this to the rapid triage and clearer communication about liver health status.
3. GP Confidence and Nurse Capability: Both patients and GPs expressed strong confidence in the nursing staff’s ability to conduct liver assessments. Many GPs felt more equipped to manage low-risk MASLD cases without specialist input, highlighting potential for task-shifting and improved efficiency.
4. Barriers to Implementation and Sustainability: The study identified persistent challenges, including:
– Variable prioritization of liver disease assessment in patients with multiple comorbidities.
– Need for further GP education in MASLD risk stratification and management pathways.
– Funding and referral infrastructure gaps for community screening programs.
– Limited access to effective diet and exercise programs for sustained lifestyle intervention.
5. Effectiveness: The model was effective in identifying high-risk MASLD patients who required specialist attention while enabling safe management of low-risk cases within primary care. This approach has potential to reduce specialist clinic burden and optimize resource allocation.
Expert Commentary
The LOCATE-NAFLD trial addresses a major bottleneck in MASLD management: the reliance on specialist clinics for risk stratification. By demonstrating the feasibility and acceptability of nurse-led, community-based screening, the trial supports a paradigm shift towards advanced primary care models. However, the results also underscore the need for integrated funding, robust referral pathways, and accessible lifestyle modification programs to ensure long-term success. Recent guidelines from the American Association for the Study of Liver Diseases (AASLD) and European Association for the Study of the Liver (EASL) emphasize multidisciplinary and community-centered approaches, aligning with LOCATE-NAFLD’s findings.
Limitations include the potential for selection bias in trial recruitment, the need for validation in diverse healthcare settings, and unresolved issues around resource allocation for community screening. Importantly, the effectiveness of nurse-led models in improving long-term clinical outcomes, such as progression to cirrhosis or cardiovascular events, warrants further study.
Conclusion
The LOCATE-NAFLD trial highlights that nurse-delivered, community-based liver screening is a promising, highly acceptable, and effective strategy for early MASLD risk identification. While the model can alleviate specialist clinic overload and empower primary care, sustainable adoption requires addressing funding, training, and infrastructure gaps, as well as improving access to lifestyle interventions. Ongoing research should focus on refining implementation in complex patient populations and evaluating long-term outcomes.
References
Allen MJ, Tulleners R, Brain D, O’Beirne J, Powell EE, Barnett A, Valery PC, Kularatna S, Hickman IJ. Implementation of a nurse-delivered, community-based liver screening and assessment program for people with metabolic dysfunction-associated steatotic liver disease (LOCATE-NAFLD trial). BMC Health Serv Res. 2025 Mar 22;25(1):421. doi: 10.1186/s12913-025-12580-5 IF: 3.0 Q2 . PMID: 40121480 IF: 3.0 Q2 ; PMCID: PMC11929169 IF: 3.0 Q2 .
Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of NAFLD: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67(1):328-357.
European Association for the Study of the Liver (EASL). EASL Clinical Practice Guidelines on non-invasive tests for evaluation of liver disease severity and prognosis. J Hepatol. 2021;75(3):659-689.