Introduction
Ulcerative colitis (UC) is a chronic inflammatory bowel disease characterized by mucosal inflammation of the colon, leading to significant morbidity and impaired quality of life. Despite advances in therapeutic options, many patients with moderate to severe UC experience relapsing disease courses, underscoring the need for effective and safe long-term treatments. Upadacitinib, a Janus kinase inhibitor, has emerged as a promising agent for inducing and maintaining remission in UC. The ongoing phase 3 U-ACTIVATE long-term extension study evaluates the durability of upadacitinib’s efficacy and safety over extended treatment durations. This article provides a detailed analysis of the interim results at 3 years, focusing on clinical and endoscopic remission rates and safety outcomes.
Study Background and Disease Burden
Ulcerative colitis affects millions worldwide with significant symptom burden including frequent diarrhea, bleeding, abdominal pain, and systemic features. Moderately to severely active disease often requires immunomodulatory or biologic therapy to achieve remission. However, loss of response and safety concerns remain challenging over prolonged treatment courses, highlighting an unmet clinical need for agents demonstrating sustained benefit and tolerability. Upadacitinib’s oral administration and selective inhibition profile offer a mechanistically novel approach with rapid onset. Previous U-ACHIEVE induction and maintenance studies established short- to medium-term efficacy and safety, prompting the U-ACTIVATE long-term extension to assess outcomes beyond one year.
Study Design
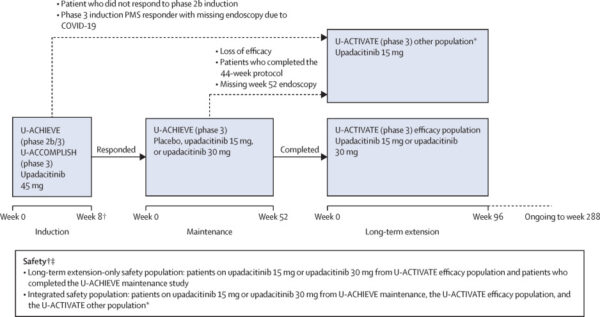
The U-ACTIVATE study is a multicenter, international, ongoing phase 3 extension trial with a total planned duration of 288 weeks across 307 centers in 43 countries. Eligible patients aged 16 to 75 years had moderately to severely active UC confirmed by adapted Mayo scores (5–9) and endoscopic subscore (2 or 3). Patients achieving clinical response after 8 weeks of induction with upadacitinib 45 mg entered the 52-week U-ACHIEVE maintenance phase. Those completing maintenance were eligible for the long-term extension (LTE) phase, continuing either 15 mg or 30 mg upadacitinib. Dose adjustments were permitted: 15 mg non-remitters could escalate to 30 mg, placebo patients could escalate to 15 mg, and 30 mg patients continued the same dose. Data from weeks 48 and 96 of LTE are presented in this interim analysis.
Key endpoints included:
– Clinical remission per adapted Mayo score
– Endoscopic remission and maintenance
– Safety based on treatment-emergent adverse events and events of special interest
Analyses used an as-observed approach; missing data were not imputed until dose switching.
Key Findings
From 414 eligible patients, 369 received upadacitinib in LTE: 142 on 15 mg and 227 on 30 mg.
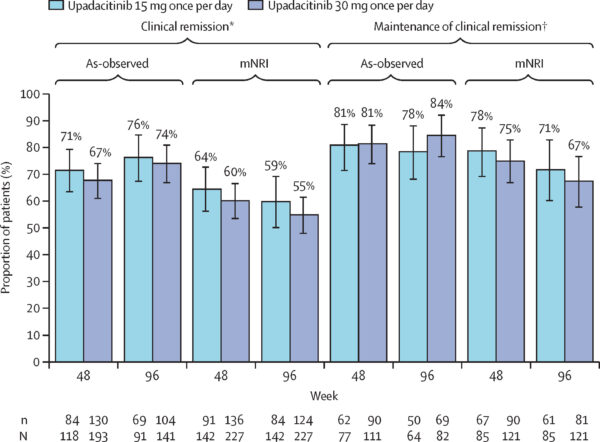
Clinical remission rates at LTE week 48 were 71% for 15 mg and 67% for 30 mg recipients; these increased slightly by week 96 to 76% and 74%, respectively. Among patients entering LTE in clinical remission, maintenance rates were high and consistent across weeks 48 and 96 (approximately 78–84%).
Endoscopic remission among observed patients was approximately 46–49% at week 48 and remained stable around 45–47% at week 96. Maintenance of endoscopic remission was sustained, with roughly 65–76% of patients maintaining remission during this period.
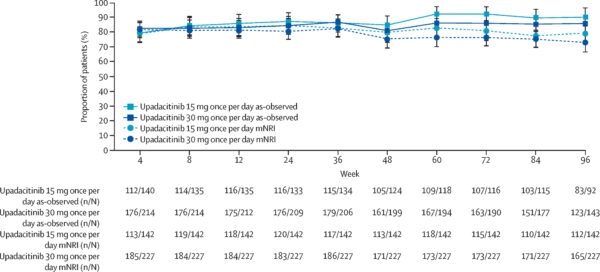
Safety data were derived from 467 patients with 1027.9 patient-years of exposure. Treatment-emergent adverse event (TEAE) rates were comparable between doses: 238.5 events/100 patient-years for 15 mg and 233.4 events/100 patient-years for 30 mg. Serious TEAE rates were 11.7 and 12.4 per 100 patient-years, respectively. Most frequent adverse events of special interest included hepatic disorders, lymphopenia, creatine phosphokinase elevation, serious infections, neutropenia, and herpes zoster. Three deaths related to TEAEs were reported over the LTE period.
These findings highlight a sustained efficacy with a manageable safety profile during prolonged upadacitinib therapy in moderate to severe UC.
Expert Commentary
The U-ACTIVATE LTE interim results reinforce upadacitinib’s role as a valuable long-term treatment option for patients with moderate to severe UC. Sustained remission rates and stable endoscopic healing strongly suggest durable mucosal control, which is critical for reducing flares and hospitalization. Safety outcomes are generally consistent with prior findings, with no unexpected adverse signals over extended treatment durations.
While the as-observed analysis underscores the treatment effect in compliant patients, limitations include potential selection bias, with patients remaining in the study possibly representing those with better disease control and tolerability. Longer follow-up and real-world data will further contextualize the benefit-risk balance.
Mechanistically, upadacitinib’s selective JAK1 inhibition may contribute to its efficacy and tailored safety profile by modulating key cytokine signaling pathways involved in UC pathogenesis.
Conclusion
This interim analysis of the U-ACTIVATE long-term extension study demonstrates that upadacitinib 15 mg and 30 mg provide sustained clinical and endoscopic remission up to nearly 3 years in patients with moderately to severely active ulcerative colitis. The favorable safety profile supports its continued use, with adverse events manageable and consistent with prior data. These findings support upadacitinib as an effective long-term therapeutic option addressing the unmet need for durable remission in UC. Ongoing data collection will inform its role in clinical practice and long-term patient outcomes.
References
Panaccione R, Vermeire S, Danese S, et al. Long-term efficacy and safety of upadacitinib in patients with moderately to severely active ulcerative colitis: an interim analysis of the phase 3 U-ACTIVATE long-term extension study. Lancet Gastroenterol Hepatol. 2025;10(6):507-519. doi:10.1016/S2468-1253(25)00017-2 IF: 38.6 Q1 .
Additional guidelines: ClinicalTrials.gov Identifier: NCT03006068.