Highlight
Monoallelic cysteine-altering NOTCH3 (NOTCH3cys) variants cause CADASIL, a well-characterized adult-onset small vessel disease, whereas biallelic NOTCH3 loss-of-function (NOTCH3lof) variants lead to a rare childhood-onset small vessel disease. This study clarifies that monoallelic NOTCH3lof variants also contribute to a distinct small vessel disease phenotype characterized by increased white matter hyperintensities but without a substantially increased stroke risk compared to controls. Cardiovascular risk factors and aging worsen clinical manifestations. Notably, vessel pathology in NOTCH3lof differs from CADASIL, especially in collagen deposition patterns.
Study Background and Disease Burden
Small vessel disease (SVD) is a leading cause of stroke and cognitive impairment worldwide. Cerebral Autosomal Dominant Arteriopathy with Subcortical Infarcts and Leukoencephalopathy (CADASIL), caused by monoallelic NOTCH3 cysteine-altering mutations, exemplifies adult-onset hereditary SVD characterized by marked white matter alterations and frequent strokes. In contrast, biallelic NOTCH3 loss-of-function mutations produce a rare childhood-onset SVD with a more severe clinical course. The clinical relevance of monoallelic NOTCH3 loss-of-function variants remains controversial, with uncertainties about their penetrance, vascular pathology, and stroke risk. Clarifying this relation is crucial for patient counseling, diagnostic interpretation of genetic findings, and personalized management strategies.
Study Design
This observational, multi-center study integrated genetic data and clinical phenotyping from diverse sources, including the Genome Aggregation Database (gnomAD), UK Biobank, six clinical centers across Europe, Asia, and the United States, and a comprehensive literature review. Allele frequency of monoallelic NOTCH3lof variants was determined from gnomAD. In UK Biobank, 102 NOTCH3lof carriers (median age 58 years, 55% female) were evaluated against NOTCH3cys carriers and matched controls for normalized white matter hyperintensity volume (nWMHv), peak width of skeletonized mean diffusivity (PSMD), lacune count, and stroke incidence. Clinically ascertained NOTCH3lof carriers (n=69) underwent neuroimaging and clinical stroke assessment, and skin biopsies were analyzed using immunohistochemistry and electron microscopy to examine vessel wall pathology.
Key Findings
Genetic Prevalence: Analysis of gnomAD identified 306 monoallelic NOTCH3lof variants, with an allele frequency of approximately 0.6 per 1,000 individuals, suggesting these variants are relatively rare but not exceptionally uncommon in the general population.
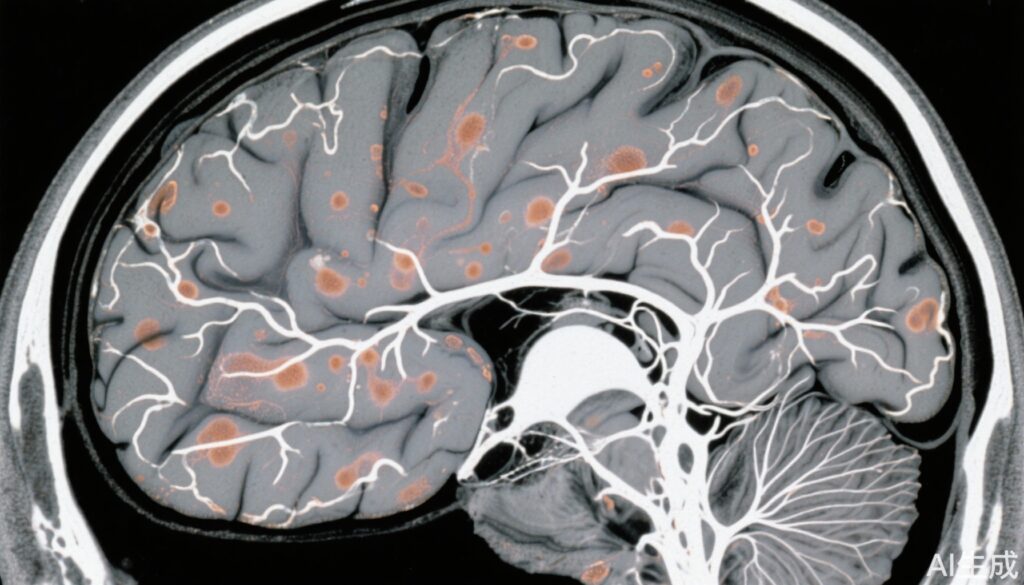
Neuroimaging Correlates: NOTCH3lof carriers exhibited significantly increased nWMHv (Δ0.44 mm³, p < 0.001) and PSMD (Δ0.19 × 10⁻⁴, p = 0.017) compared to controls, indicating white matter microstructural abnormalities and small vessel involvement. These measures were statistically comparable to NOTCH3cys CADASIL cases, highlighting shared pathophysiological substrate regarding white matter changes.
Stroke Risk: Unlike NOTCH3cys carriers who demonstrated a heightened stroke risk, NOTCH3lof carriers did not display a significantly increased stroke incidence relative to controls. Clinically ascertained NOTCH3lof individuals had a stroke prevalence of 15%, often correlating with advancing age and presence of cardiovascular risk factors.
Clinical Phenotype: Among clinical cases, 88% had white matter hyperintensities, while lacunes were present in 38%, often in those with cardiovascular comorbidities. Clinical manifestations appeared largely subclinical until exacerbated by risk factors and aging.
Vessel Pathology: Skin biopsies from NOTCH3lof individuals demonstrated significantly more abundant collagen deposition in vessel walls compared to both NOTCH3cys cases (37% vs 10%; p = 0.016) and controls (37% vs 5%; p < 0.001). This suggests a distinct vascular remodeling pattern divergent from the granulovascular changes prominent in CADASIL.
Expert Commentary
This investigation resolves an important clinical ambiguity by establishing that monoallelic NOTCH3 loss-of-function mutations, previously of uncertain pathogenic significance, are associated with a distinct SVD phenotype. The absence of excess stroke risk in this group contrasts with CADASIL, despite comparable neuroimaging burden, implying differing mechanisms and clinical trajectories. The prominent collagen deposition in vessel walls hints at distinct pathobiology, potentially amenable to targeted therapeutic strategies. These findings emphasize the multifactorial nature of clinical expression, where genotype interacts with traditional vascular risk factors and aging. Clinicians should cautiously interpret NOTCH3lof variant findings, avoiding deterministic prognostications, and focus on optimizing vascular risk management.
Limitations include observational design and relatively limited longitudinal data on clinical progression or cognitive outcomes. Further studies could clarify mechanistic pathways, long-term prognosis, and potential interventions.
Conclusion
Monoallelic NOTCH3 loss-of-function variants induce a mild, largely subclinical small vessel disease characterized by white matter abnormalities and distinctive vascular collagen remodeling. This phenotype differs fundamentally from CADASIL in pathology and stroke risk. Identification of these variants warrants careful clinical evaluation and comprehensive vascular risk factor management rather than aggressive CADASIL-like treatment. This nuanced understanding enhances genetic counseling and informs personalized surveillance strategies, addressing an important unmet need in hereditary small vessel disease diagnostics and care.
References
van Asbeck JS, Gravesteijn G, Cerfontaine MN, et al. Small Vessel Disease Phenotype Associated With Monoallelic NOTCH3 Loss-of-Function Variants. Neurology. 2025 Sep 23;105(6):e214021. doi: 10.1212/WNL.0000000000214021.
Additional references for broader context:
– Chabriat H, Joutel A, Dichgans M, et al. CADASIL. Lancet Neurol. 2009;8(7):643-653.
– Markus HS. Cerebral small vessel disease pathogenesis and clinical characteristics. Lancet Neurol. 2017 Mar;16(7):934-947.