Study Background and Disease Burden
Chronic heart failure (HF) remains a leading cause of morbidity and mortality globally, imposing significant health and economic burdens. Despite advances in pharmacological and device therapies, mortality rates remain high, particularly in patients hospitalized with worsening symptoms. Hemodynamically guided management through ambulatory monitoring of pulmonary artery (PA) pressures using implantable remote sensors has emerged as a promising strategy. Prior randomized trials demonstrated that reducing elevated PA pressures can lower mortality and hospitalization rates in patients with New York Heart Association (NYHA) class III HF. However, several fundamental questions remain unanswered: which PA pressure measurements (systolic, diastolic, or mean) most accurately predict all-cause mortality? Are these predictive across the spectrum of left ventricular ejection fractions (EFs), including both reduced (<50%) and preserved (≥50%) EF? Additionally, do temporal changes in PA pressure relate to subsequent survival outcomes? Addressing these questions can refine risk stratification and optimize therapeutic interventions in HF management.
Study Design
This comprehensive retrospective analysis pooled data from four major clinical studies involving the CardioMEMS HF System—a remote pulmonary artery pressure monitoring device. The combined cohort included 4342 patients with chronic HF from CHAMPION (n=550), GUIDE-HF (n=2358), US Post Approval Study (US PAS; n=1200), and MEMS-HF (n=234), regardless of treatment assignment. Patients were categorized by EF into reduced EF (<50%, n=2562) and preserved EF (≥50%, n=1454) groups. PA systolic, diastolic, and mean pressures were assessed at baseline—defined as the average pressure over 14 days following implantation—and changes were evaluated from baseline to 6 months (again averaged over 14 days just before month 6). The primary endpoint was all-cause mortality assessed over a 2-year observation period. Statistical analyses included continuous and categorical models to quantify hazard ratios (HRs) for mortality risk associated with baseline and longitudinal changes in PA pressures.
Key Findings
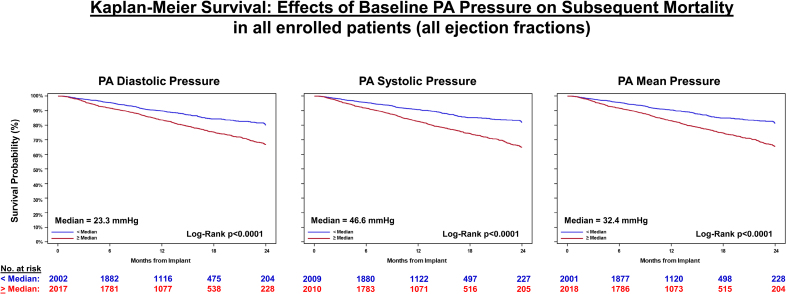
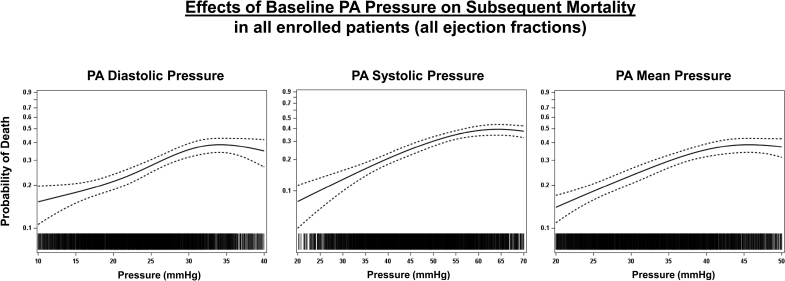
The analyzed population had median follow-up durations ranging 12 to 22.8 months. Of 4019 patients with follow-up data, 682 (approximately 17%) died over 2 years. Mortality risk exhibited a nearly linear increase with higher baseline PA pressures across all types—systolic, diastolic, and mean—and conversely lower risk at lower pressures. This pattern held true when analyzed above or below the median and across physiological ranges (e.g., diastolic PA pressure 15 to 35 mmHg).
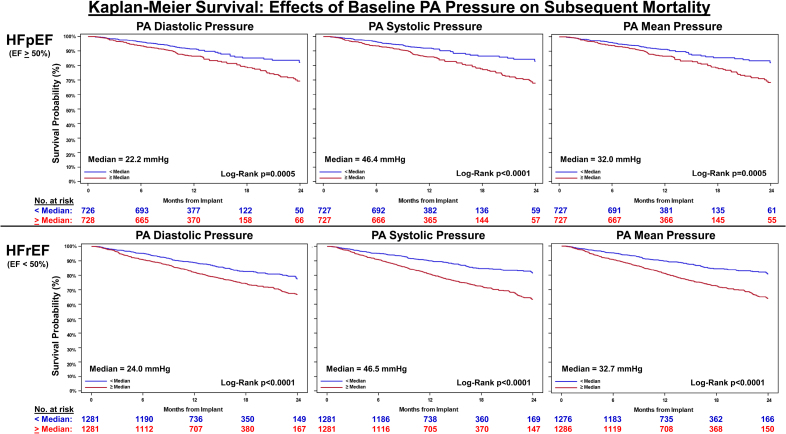
Notably, these associations were consistent in both EF categories: patients with HFpEF had a 25.1% mortality rate, and those with HFrEF had a 28.0% rate at 2 years.
Kaplan-Meier survival: effects of baseline pulmonary artery (PA) pressure on subsequent mortality in all enrolled patients (all ejection fractions).
Effects of baseline pulmonary artery (PA) pressure on subsequent mortality in all enrolled patients (all ejection fractions).
Kaplan-Meier survival: effects of baseline pulmonary artery (PA) pressure on subsequent mortality.
Multivariate analysis identified baseline PA diastolic pressure as an independent predictor of mortality (HR 1.04 per mmHg increase, 95% CI 1.03-1.05; P2 mmHg decrease in PA diastolic pressure was associated with a 14.7% reduction in mortality, while a >2 mmHg increase predicted a 26.7% rise in mortality (P=0.0237).
Systolic and mean PA pressures at baseline and their changes over 6 months also demonstrated statistically significant prediction of mortality across EF subgroups. For categorical analyses, thresholds for significant pressure changes were set at 2 mmHg for diastolic and mean, and 3 mmHg for systolic pressures due to measurement variability.
Forest plot analyses confirmed consistent hazard ratios >1.0 (indicating increased risk) for PA pressures in both preserved and reduced EF groups, with all P-values <0.05 except for borderline significance in some markers within HFrEF.
Expert Commentary
This large-scale pooled analysis reinforces the prognostic value of ambulatory pulmonary artery pressure monitoring in chronic HF, highlighting that baseline and dynamic changes in PA pressures predict all-cause mortality irrespective of EF category. The findings suggest that not only are elevated pressures deleterious, but that trends toward improvement or worsening in PA pressures over a relatively short period (6 months) carry significant prognostic weight.
These results align with current heart failure guidelines that emphasize tailored management, potentially enabling more precise hemodynamic optimization. The robustness across EF groups is particularly valuable for HFpEF patients, who have fewer proven therapeutic options and for whom risk prediction remains challenging.
One limitation is the retrospective design and heterogeneity across source studies, although inclusion criteria and monitoring technology were consistent. These findings warrant prospective validation and exploration of how integrating PA pressure trajectories into clinical decision-making impacts patient-centered outcomes.
Conclusion
Baseline pulmonary artery systolic, diastolic, and mean pressures are significant independent predictors of 2-year all-cause mortality in patients with chronic heart failure. Importantly, changes in these pressures from baseline to 6 months also independently predict survival outcomes, underscoring the dynamic nature of hemodynamic risk. These relationships hold true across the spectrum of left ventricular ejection fraction, reinforcing the utility of remote ambulatory PA pressure monitoring for comprehensive risk stratification and management guidance in chronic HF.
References
1. Zile MR, Abraham WT, Stevenson LW, et al. Relationship Between Remote, Ambulatory Pulmonary Artery Pressures, and All-Cause Mortality in Patients With Chronic Heart Failure. Circulation: Heart Failure. 2025;18(6):e012754. doi:10.1161/CIRCHEARTFAILURE.124.012754 IF: 8.4 Q1 .
2. Abraham WT, Adamson PB, Bourge RC, et al. Wireless pulmonary artery haemodynamic monitoring in chronic heart failure: a randomized controlled trial. Lancet. 2011;377(9766):658-666.
3. Lindenfeld J, Zile MR, Desai AS, et al. Haemodynamic-GUIDEd management of Heart Failure (GUIDE-HF): Design and baseline characteristics. ESC Heart Fail. 2021;8(6):4994-5004.
4. McMurray JJ, Packer M, Desai AS, et al. Angiotensin–Neprilysin inhibition versus enalapril in heart failure. New England Journal of Medicine. 2014;371(11):993-1004.