Highlights
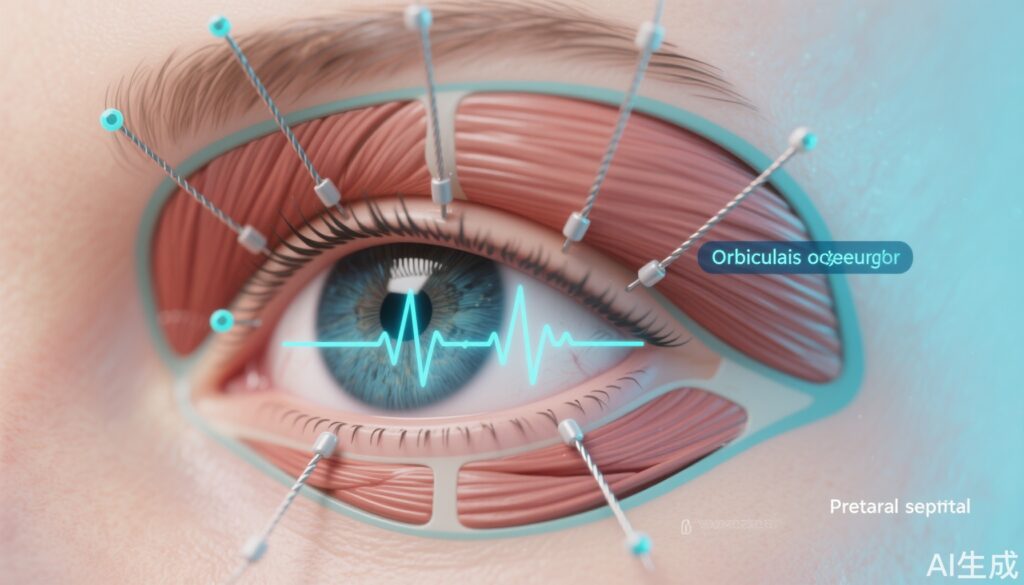
– UCLA investigators used intramuscular wire EMG and high-speed motion capture to show that the orbicularis oculi executes segmental, task-specific activation patterns rather than a unitary up–down contraction.
– Five eyelid closure behaviors (spontaneous, voluntary, reflexive, soft closure, forced closure) differ in onset timing, spatial recruitment within the muscle, and kinematic trajectory.
– The findings provide practical guidance for electrode placement, timing strategies, and stimulation design for future blink-assisting neuroprostheses aimed at preventing exposure keratopathy in patients with eyelid paralysis.
Study background and disease burden
Blinking is a rapid, regularly occurring motor behavior that maintains ocular surface homeostasis by spreading tear film, clearing debris, and protecting the cornea. Loss of effective eyelid closure—whether from facial nerve palsy (Bell palsy, traumatic/iatrogenic injury), central lesions (stroke), neoplasm, infection, or neuromuscular disease—leads to exposure keratopathy, chronic irritation, corneal ulceration, and ultimately vision loss if untreated. Clinically available measures to reduce corneal exposure include lubricating drops/ointments, moisture chambers, botulinum toxin-induced ptosis, temporary or permanent tarsorrhaphy, and surgical insertion of eyelid weights. Each has limitations: many are either palliative, cosmetically undesirable, or compromise vision.
Functional electrical stimulation (FES) and implantable neurostimulation have restored function in other paralyzed muscle groups (e.g., diaphragm pacing, sacral nerve stimulation). However, the eyelid presents unique challenges: the orbicularis oculi is anatomically thin, has fine motor control and rapid timing demands, and generates both protective reflexive and baseline maintenance blinks. Designing a reliable blink neuroprosthesis requires detailed knowledge of how the orbicularis oculi is neurally controlled across behaviors—information that, until now, has been incomplete.
Study design
The study by Kim et al. (Kim J., et al. 2025, Proceedings of the National Academy of Sciences) used an experimental design in healthy adult volunteers to characterize muscle activation and eyelid kinematics across multiple blink behaviors. An ophthalmic surgeon inserted fine wire electrodes into the orbicularis oculi to record intramuscular electromyographic (EMG) signals with high spatial resolution. Simultaneously, a motion-capture system recorded eyelid movement in ultraslow motion, enabling precise measurement of onset timing, velocity, trajectory, and the spatial origin of movement.
Five closure behaviors were elicited and recorded: spontaneous (unprompted baseline blinks), voluntary (on command), reflexive (air puff/collision-protective stimuli), soft closure (slow, gentle descent as in drowsiness), and forced closure (strong, tightly squeezed eyelid closure). The primary endpoints included spatial-temporal EMG activation patterns within muscle segments, onset latencies, kinematic profiles of the upper and lower eyelids, and correlations between regional EMG activity and specific phases of eyelid motion.
Key findings
The principal result is that human eyelid behavior is driven by segmental neural control of the orbicularis oculi, not by a single homogeneous contraction. Specific points:
– Segmental recruitment: Different regions of the orbicularis oculi (for example, pretarsal vs preseptal segments and medial vs lateral portions) were preferentially activated for different blink types. Spontaneous blinks tended to recruit a stereotyped short-latency pretarsal activation, whereas forced closures produced broad, near-synchronous recruitment across multiple segments.
– Temporal sequencing: Protective reflexive blinks exhibited extremely rapid, centrally initiated activation sequences with minimal delay between segments, consistent with a brainstem reflex brigade designed to protect the globe. Voluntary blinks showed longer and more variable onset latencies with different initiation sites depending on subject strategy. Soft closures were characterized by slow ramping activation in a limited subset of muscle fibers.
– Kinematic complexity: Motion capture showed that eyelid closure is not purely vertical. Segmental activation produced subtle changes in lid contour, medial–lateral translation, and rotational components that likely affect tear film distribution and corneal coverage. Reflexive closures were fastest and most compressive; spontaneous blinks had brief closure duration optimized for tear film maintenance.
– Correlation of EMG and motion: The precise timing of regional EMG bursts predicted local eyelid motion; early activation in a medial pretarsal sector produced medial lid descent before lateral segments engaged. This finding supports the hypothesis that targeted stimulation of specific orbicularis oculi sectors could reproduce natural blink kinematics.
– Practical implications for stimulation: Based on activation maps, the authors identified candidate electrode target zones and timing windows that would be most efficient to produce either protective rapid closure or routine lubrication blinks. They suggest that a multi-contact electrode array with time-sequenced pulses could mimic natural patterns more closely than single-site stimulation.
While the publication focuses on healthy volunteers, the authors explicitly frame the data as a template for designing clinical neuroprostheses for patients who have lost effective blinking.
Expert commentary and interpretation
The study provides a technically rigorous and clinically relevant advance in understanding eyelid biomechanics and neural control. For clinicians and device developers, several practical insights emerge:
– Electrode placement matters: Pretarsal activation is critical for rapid, protective closure; preseptal and lateral regions contribute to lid contouring. Implant designs should consider multi-contact leads that can selectively stimulate these regions rather than a single electrode in a bulk muscle belly.
– Timing is as important as amplitude: Protective blinks require very short, precisely timed pulses across segments. A neuroprosthesis will likely need millisecond-level coordination and either a reliable trigger signal (e.g., corneal proximity sensor, eyelid position feedback) or integration with brainstem reflex pathways (challenging clinically).
– Behavior-specific stimulation programs: Because spontaneous and reflexive blinks differ in recruitment and timing, stimulation paradigms should be behavior-specific (e.g., low-intensity periodic pulses for lubrication vs high-intensity, synchronous pulses for protective closure).
– Translational hurdles remain: Healthy muscle recruitment dynamics differ from chronically denervated or atrophic muscle, where motor endplate loss, fibrosis, and synkinesis may alter stimulation thresholds and selectivity. The presence of synkinetic reinnervation after facial nerve injury could cause unwanted contractions.
– Safety and comfort: The eyelid is a sensitive area. Implantable systems must minimize tissue irritation, infection risk from transcutaneous leads, and patient discomfort from suprathreshold stimulation.
Limitations of the study include its experimental setting in healthy volunteers and the invasive electrode method that may not be tolerable in broader populations. The sample size and demographics (not detailed here) may limit generalizability across ages and disease states. Importantly, pathological muscles may require different stimulation strategies because of altered biomechanics and electrical properties.
Clinical and device-development implications
For clinicians managing eyelid paralysis, the study clarifies why some interventions are imperfect and where next-generation solutions might improve outcomes. A successful blink neuroprosthesis would ideally:
– Restore regular lubrication blinks to prevent chronic exposure keratopathy and reduce need for frequent topical lubrication.
– Provide rapid, protective closure on demand or when sensors detect an imminent threat to the globe.
– Be cosmetically acceptable, durable, and programmable to individual patient needs.
Development pathway considerations:
– Preclinical testing should include chronic stimulation in animal models or cadaveric tissue to assess electrode stability, tissue response, and stimulation thresholds over time.
– Early feasibility human studies could target patients with severe exposure keratopathy refractory to conventional measures; endpoints would include objective eyelid closure metrics, corneal staining scores, tear film parameters (TBUT), and patient-reported ocular comfort and vision-related quality of life.
– Regulatory pathway: device sponsors should engage early with regulatory bodies (e.g., FDA) to define appropriate premarket studies and whether a de novo or IDE-supported trial is necessary.
Safety, ethical and practical considerations
Key safety issues include infection risk, lead migration, unintended muscle activation (e.g., synkinesis or spread to facial expression muscles), and potential interference with vision during forced closures. Ethically, interventions aimed at facial restoration must weigh functional benefit against risks of implant surgery and the psychosocial value of more natural eyelid movement. Patient selection will be critical: those with intact lower motor neurons but loss of central control (e.g., selective lesions) might be better candidates than those with end-stage denervation.
Unanswered questions and next steps
Important areas for future research include:
– Validation in patients with facial paralysis and chronic exposure keratopathy to determine how disease-altered tissue responds to patterned stimulation.
– Development of multi-contact, miniaturized electrode arrays and controller algorithms that can deliver behavior-specific stimulation patterns identified by the UCLA team.
– Integration of closed-loop sensing (lid position, corneal moisture, blink frequency detectors) to adapt stimulation in real time.
– Long-term safety and durability studies addressing tissue response and device maintenance.
Conclusion
The UCLA study provides a high-resolution map of how the human orbicularis oculi is recruited across different eyelid behaviors and offers a practical blueprint for designing blink-assisting neuroprostheses. For clinicians, the data underscore why simple single-site stimulation has been insufficient and point toward multi-site, time-sequenced approaches that more faithfully replicate natural blink kinematics. The translational path will require engineering solutions for selective stimulation, careful human trials in patients with eyelid paralysis, and regulatory navigation, but the work represents a substantial step toward restoring a small yet critically important motor behavior with major implications for ocular health.
References
Kim J., et al. Human eyelid behavior is driven by segmental neural control of the orbicularis oculi. Proceedings of the National Academy of Sciences. 2025. doi.org/10.1073/pnas.2508058122