Highlight
– Use of a customized protective palatal obturator (CPPO) eliminates oral tissue injury in newborns during intubation for cleft lip surgery.
– CPPO does not increase the intubation time or the number of intubation attempts compared to standard procedures.
– The device demonstrates potential for facilitating safer airway management in neonates with orofacial clefts, especially severe types.
– This is the first randomized trial assessing CPPO efficacy in neonatal cleft lip surgery intubation.
Study Background
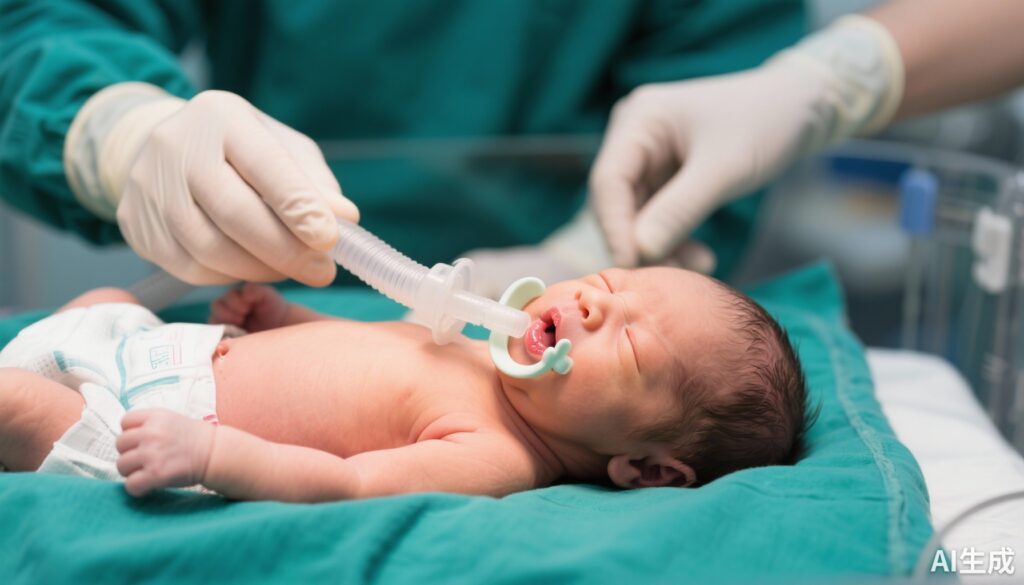
Orofacial clefts, including cleft lip and palate, are among the most common congenital craniofacial anomalies worldwide. These defects not only compromise facial aesthetics but also challenge vital functions such as feeding and airway management. Newborns requiring surgical correction of cleft lip often mandate general anesthesia with endotracheal intubation. Due to the anatomical disruption caused by the cleft, intubation can be technically difficult and is associated with increased risk of oral mucosal injury, bleeding, and other complications.
Protective strategies to minimize trauma during intubation are thus clinically important in this vulnerable population. The customized protective palatal obturator (CPPO) was developed to provide an anatomical barrier between the fragile oral tissues and the intubation equipment, potentially reducing mucosal injuries and facilitating the intubation process.
Study Design
This was a single-center, randomized controlled neonatal sub-study involving 55 newborns scheduled for cleft lip repair surgery. Participants were randomized into two groups: the intervention group received intubation with a CPPO in place, and the control group underwent standard intubation without this device. The study was registered in ClinicalTrials.gov under identifiers NCT04422847 and NCT04422964.
The primary endpoint was the presence, severity, and anatomical location of oral tissue trauma incurred during intubation. Secondary endpoints included intubation difficulty assessed by modified Cormack-Lehane scoring during laryngoscopy, total intubation time, number of intubation attempts, and anesthesiological complications.
Key Findings
The intervention group (CPPO) demonstrated a complete absence of oral tissue damage associated with intubation, while the control group experienced a 21.4% incidence of tissue injury. This difference was statistically significant (p = .023), highlighting a clear protective effect of the CPPO device.
Intubation time and the number of attempts required were similar between groups, indicating that CPPO use did not prolong or complicate airway access. Although difficult intubations occurred less frequently in the CPPO group (40.7%) compared to controls (50%), this difference did not reach statistical significance.
Importantly, no increase in anesthesiologic complications related to CPPO use was observed, supporting its safety profile. The device appeared particularly advantageous in cases of more severe clefts, which present the greatest technical challenges for airway management.
Expert Commentary
The findings from this rigorously conducted randomized controlled trial provide compelling evidence for the clinical benefit of a customized protective palatal obturator in neonates with cleft lip undergoing intubation. By eliminating oral mucosal injuries, CPPO use can improve procedural safety and potentially reduce associated morbidity, such as bleeding and secondary infection risks.
The absence of delay in intubation time and the unchanged number of attempts further validate the device’s ease of use in a high-risk population. While the reduction in difficult intubations did not reach statistical significance, the trend suggests that CPPO may contribute to easier laryngoscopy visualization by stabilizing palatal anatomy.
Still, generalizability to other centers and broader neonatal populations requires cautious optimism. The single-center design and modest sample size warrant further multicenter studies. Moreover, longer-term postoperative outcomes related to CPPO use remain to be assessed.
Conclusion
The customized protective palatal obturator is an effective and safe innovation that significantly reduces intubation-related oral tissue injury in newborns undergoing cleft lip surgery. It offers a practical advance in neonatal airway management without compromising procedural efficiency. Given its particular benefits in severe cleft cases, integrating CPPO into anesthetic protocols may define a new standard of care in specialized craniofacial surgery centers.
Funding and Clinical Trial Registration
This study was supported by institutional funding from the participating medical center. Both clinical trials were registered on ClinicalTrials.gov (NCT04422847 and NCT04422964).
References
Richtrová M, Košková O, Marcián P, Borák L, Bönischová T, Fabián D, Janků M, Joukal M, Vymazalová K, Štourač P. Customized protective palatal obturator for intubation in newborns in cleft lip surgery: a randomized controlled trial. Ann Med. 2025 Dec;57(1):2561802. doi: 10.1080/07853890.2025.2561802. Epub 2025 Sep 22. PMID: 40981509; PMCID: PMC12456041.