Highlight
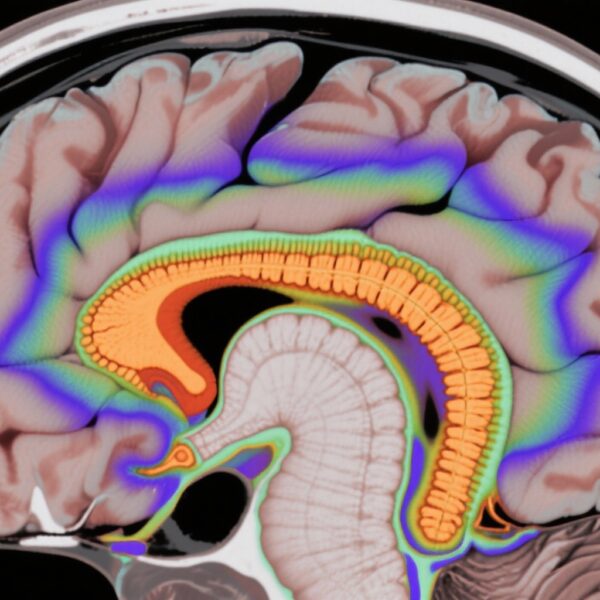
- Amygdala tau accumulation correlates with increasing depressive symptoms in cognitively normal older adults.
- APOE ε4 carriers exhibit stronger associations between tau pathology and depressive symptom progression.
- Entorhinal tau primarily associates with memory decline, distinct from depressive symptoms linked to amygdala tau.
- Longitudinal PET imaging with [18F]Flortaucipir enables tracking tau pathology’s contribution to neuropsychiatric trajectories.
Background
Depressive symptoms rise in prevalence with advancing age and substantially impact quality of life and functional outcomes, even among individuals without overt cognitive impairment. The neuropathological mechanisms underlying late-life depression remain incompletely understood. Emerging evidence points to tau protein accumulation—a hallmark of Alzheimer’s disease (AD) pathology—not only in traditionally studied brain regions but also within limbic structures such as the amygdala.
The amygdala plays a crucial role in emotional regulation and processing. Tau aggregation in this region could disrupt neural circuits implicated in mood regulation, potentially contributing to depressive symptomatology in aging populations. Furthermore, genetic risk factors such as the apolipoprotein E (APOE) ε4 allele may modify these pathological effects, enhancing vulnerability to neuropsychiatric changes. Understanding the interplay among tau pathology in the amygdala, genetic predisposition, and clinical symptoms is critical for early identification and targeted interventions.
Study Design and Methods
This investigation utilized data from the Alzheimer’s Disease Neuroimaging Initiative (ADNI), a multicenter longitudinal cohort designed to study biomarkers and clinical progression in aging and AD. The main study population comprised cognitively normal older adults with baseline and follow-up assessments.
Tau pathology was quantified cross-sectionally and longitudinally using [18F]Flortaucipir (FTP) positron emission tomography (PET) focusing primarily on the amygdala and entorhinal cortex regions. Depressive symptoms were evaluated using standardized clinical scales assessed over time. The study design incorporated assessments of memory function and considered amyloid beta status along with APOE ε4 genotype to explore potential moderating effects.
Secondary analyses were performed with the Berkeley Aging Cohort Study (BACS) to replicate findings and validate observed associations.
Key Findings
The study demonstrated that longitudinal increases in depressive symptoms were significantly associated with higher baseline tau deposition and increasing tau accumulation detected in the amygdala by FTP PET imaging. The magnitude of this relationship was particularly pronounced in individuals carrying the APOE ε4 allele, suggesting a gene-pathology interaction.
Interestingly, while entorhinal cortex tau accumulation was associated with memory decline, it did not contribute significantly to depressive symptoms beyond the variance explained by amygdala tau levels. These findings indicate a region-specific impact of tau pathology on clinical phenotypes: the amygdala’s involvement in neuropsychiatric symptoms and the entorhinal cortex’s role in cognitive decline.
Replication of APOE ε4’s moderating effect on tau-depression associations in the BACS cohort underscores the robustness and generalizability of the findings.
Expert Commentary
This study advances our understanding of the neuropathological substrates underpinning late-life depressive symptoms by highlighting the critical role of amygdala tau pathology. The observed selective vulnerability of the amygdala to tau-related neuropsychiatric manifestations offers a mechanistic linkage between molecular pathology and mood disturbances.
The stronger coupling in APOE ε4 carriers aligns with previous literature indicating that ε4 status exacerbates tau-associated neurodegeneration and symptomatology. Clinically, these findings support the inclusion of tau PET imaging biomarkers in the assessment of aging individuals presenting with depressive symptoms to enhance diagnostic precision and potentially stratify risk for subsequent cognitive decline.
Notwithstanding, the study’s limitations include the observational design, which precludes causal inferences, and the reliance on PET imaging that, while sensitive, may not capture all aspects of tau pathology dynamics. Additionally, heterogeneity in depressive symptom assessment tools could affect measurement sensitivity. Further longitudinal studies integrating multimodal biomarker profiles and intervention trials will be critical to validating these findings and translating them into clinical practice.
Conclusion
The accumulation of tau pathology in the amygdala emerges as a significant contributor to escalating depressive symptoms among cognitively normal older adults, with APOE ε4 genotype amplifying this association. These insights underscore the importance of targeting amygdala tau pathology in research and therapeutic strategies aimed at mitigating neuropsychiatric changes in aging. Early detection and intervention could ultimately modify disease trajectories and improve quality of life in this vulnerable population.
Funding and Clinical Trials
The data analyzed in this work were provided by the Alzheimer’s Disease Neuroimaging Initiative (ADNI), a public-private partnership funded by NIH grants, pharmaceutical companies, and non-profit organizations. Details on clinical trials and cohort information are accessible via the ADNI database.
References
Markova TZ, Fonseca CS, Ciampa CJ, Murphy A, Landau S, Harrison TM, Berry AS; Alzheimer’s Disease Neuroimaging Initiative. Defining the contributions of tau pathology in the amygdala to increasing depressive symptoms in aging. Alzheimers Dement. 2025 Oct;21(10):e70740. doi: 10.1002/alz.70740. PMID: 41030130; PMCID: PMC12484709.
Jack CR Jr, et al. NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimers Dement. 2018;14(4):535-562.
Zhou Y, et al. Amygdala Tau Pathology and Symptomatology in Alzheimer’s Disease. Nat Neurosci. 2021;24(5):683-692.