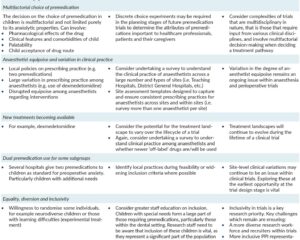
Introduction and Context
The American Diabetes Association (ADA) annually publishes the Standards of Care in Diabetes—an authoritative, evidence-graded consensus that guides screening, diagnosis, therapy, and monitoring for people with diabetes. The 2024 edition (Diabetes Care 2024;47 Suppl 1) updates prior recommendations to reflect a rapidly changing therapeutic environment: broader use of continuous glucose monitoring (CGM), expanded indications and outcome data for SGLT2 inhibitors and GLP‑1 receptor agonists, and the emergence of potent dual incretin (GIP/GLP‑1) agonists and new weight‑management approvals.
This update matters because the last several years have produced large outcome trials and new regulatory approvals that change who benefits from specific drug classes (cardio‑renal protection independent of glucose lowering), and because obesity pharmacotherapy has matured into a mainstream tool that intersects tightly with diabetes prevention and treatment. The ADA 2024 Standards synthesize this evidence into clinical practice guidance that emphasizes individualized goals, comorbidity‑driven therapy selection, and greater use of technology.
New Guideline Highlights
– Screening: The ADA continues its pragmatic approach to broaden case‑finding. Screening is recommended for adults starting at age 35 regardless of weight, and earlier for those with risk factors. Early detection is emphasized because of high prevalence of undiagnosed disease and the availability of effective therapy to reduce complications.
– Pharmacologic strategy: The Standards place comorbidity (atherosclerotic cardiovascular disease [ASCVD], heart failure, chronic kidney disease [CKD]) at the center of initial noninsulin pharmacologic choices, particularly recommending SGLT2 inhibitors and GLP‑1 receptor agonists with proven outcome benefits when those comorbidities are present—often independent of baseline HbA1c.
– Weight management: New guidance integrates anti‑obesity medications (AOMs) such as GLP‑1 agonists and dual GIP/GLP‑1 agonists into diabetes care. The Standards acknowledge the powerful weight‑loss effects of agents like semaglutide and tirzepatide and their role in both prevention and treatment.
– Technology: CGM is recommended more broadly, including for many people with type 2 diabetes (T2D) treated with insulin and even for selected patients on noninsulin regimens to support glycemic optimization and hypoglycemia reduction.
– Prevention: Lifestyle modification remains first line for prediabetes; metformin remains an option for prevention in selected higher‑risk groups (BMI ≥35 kg/m2, history of gestational diabetes, age <60 years).
Key takeaways for clinicians
– Think beyond glycemic control: choose drugs that address cardiorenal risk where indicated.
– Offer obesity treatment as an integrated part of diabetes care.
– Use CGM more widely to guide therapy and reduce hypoglycemia.
Updated Recommendations and Key Changes
Below are core changes compared with prior editions, with the ADA evidence‑grade framework (A = clear evidence from randomized controlled trials or meta‑analyses; B = supportive evidence from randomized trials or well‑conducted cohort studies; C = supportive evidence from nonrandomized studies; E = expert consensus or clinical experience).
Major updates (side‑by‑side summary):
– Screening age: continues to recommend starting at age ≥35 (ADA change introduced earlier, reaffirmed in 2024) (Grade B).
– Comorbidity‑driven therapy: stronger emphasis on starting SGLT2 or GLP‑1 with proven outcomes in patients with ASCVD, HF, or CKD, even if HbA1c is near goal (Grade A for many recommendations based on cardiovascular and renal outcome trials).
– Weight‑loss pharmacotherapy: explicit inclusion of approved anti‑obesity medications (including semaglutide and tirzepatide) as treatment options for people with T2D and obesity (Grade A–B depending on indication and trial evidence).
– CGM: widened recommendations for CGM use in T2D on insulin and selected noninsulin regimens; CGM metrics (time in range, time below range) emphasized alongside HbA1c (Grade A–B).
– Dual incretin agents: the Standards incorporate evidence on tirzepatide’s superior glucose lowering and weight loss versus some GLP‑1s and note the need for longer‑term outcome data for cardiovascular endpoints (Grade B to E pending long‑term data).
Why the updates? Driving evidence includes large randomized outcome trials demonstrating cardiovascular and renal benefits for SGLT2 inhibitors (EMPA‑REG, DAPA‑HF/DAPA‑CKD) and several GLP‑1 receptor agonists (LEADER, REWIND), as well as recent trials showing robust glycemic and weight benefits for newer agents (e.g., tirzepatide and high‑dose semaglutide). In parallel, real‑world data and device improvements have made CGM more practical and informative for broader patient populations.
Topic-by-Topic Recommendations
Diagnosis and screening
– Diagnostic criteria remain unchanged: HbA1c ≥6.5%, fasting plasma glucose ≥126 mg/dL (7.0 mmol/L), 2‑hour plasma glucose ≥200 mg/dL on 75‑g OGTT, or random plasma glucose ≥200 mg/dL with symptoms.
– Screening: adults aged ≥35 years routinely; younger adults who are overweight/obese with additional risk factors; pregnant and postpartum screening as per pregnancy‑specific guidance (24–28 weeks for GDM screening), and targeted screening for children/adolescents with risk factors.
Glycemic targets and monitoring
– Individualize HbA1c goals: many nonpregnant adults aim for <7.0% (53 mmol/mol) (Grade A), while tighter (70% and time <70 mg/dL <4% (Grade A). The Standards recommend wider use of CGM to reduce hypoglycemia and personalize therapy.
Pharmacologic therapy—principles
– Base initial and subsequent pharmacologic choices on a combination of individualized HbA1c goals, side‑effect profile, patient preferences, cost/access, and the presence of ASCVD, heart failure, or CKD (Grade A–B).
– Metformin remains first‑line for many patients with T2D when tolerated (Grade A) unless contraindicated.
When comorbidities are present
– ASCVD: consider adding a GLP‑1 receptor agonist with proven cardiovascular benefit (e.g., liraglutide, semaglutide, dulaglutide depending on evidence) or an SGLT2 inhibitor with proven benefit (Grade A).
– Heart failure (particularly heart failure with reduced ejection fraction): an SGLT2 inhibitor with proven HF benefit is recommended (Grade A), independent of baseline HbA1c for HF risk reduction.
– Chronic kidney disease: SGLT2 inhibitors with kidney benefit are recommended to slow progression and reduce events (Grade A), again independent of baseline glycemic control in many situations.
Use of GLP‑1 receptor agonists and dual agonists
– GLP‑1 receptor agonists with proven cardiovascular benefit should be prioritized in patients with ASCVD (Grade A).
– Dual GIP/GLP‑1 agonists (tirzepatide) produce large reductions in HbA1c and body weight and are included as options; the ADA underscores impressive efficacy but notes ongoing work to define long‑term cardiovascular outcomes and long‑term safety (Grade B to E).
SGLT2 inhibitors
– Recommended in patients with type 2 diabetes and CKD, HF, or high risk of ASCVD for cardiorenal protection (Grade A). Monitor volume status and renal function; counsel about genital mycotic infections and rare risks (e.g., euglycemic DKA in insulin‑deficient states).
Obesity and weight‑management drugs
– The ADA explicitly integrates AOMs as part of diabetes management when indicated: offer lifestyle therapy plus pharmacotherapy for appropriate patients with overweight/obesity, and consider AOMs for weight reduction to improve glycemic and cardiometabolic outcomes (Grade A–B). The Standards call attention to the weight loss achieved with semaglutide and tirzepatide and recommend discussing expectations, side effects, and cost/coverage with patients.
Insulin therapy
– Insulin remains the most effective glucose‑lowering therapy and is required for type 1 diabetes and for many with type 2 in advanced disease. Initiation and intensification should be individualized; combination strategies (GLP‑1 plus basal insulin) can reduce required insulin doses and weight gain (Grade A–B).
Special populations
– Pregnancy: the Standards reiterate pregnancy‑specific glycemic targets and the importance of preconception glycemic optimization. Many GLP‑1s and SGLT2s are not recommended in pregnancy.
– Older adults and frail patients: prioritize safety and avoidance of hypoglycemia. Less stringent HbA1c targets are acceptable when life expectancy or frailty limits benefit (Grade A–E).
– Pediatrics: lifestyle remains primary; pharmacologic choices vary by age and approval status; insulin and metformin remain core agents for youth with diabetes.
Follow-up and monitoring
– Regular monitoring for complications (retinopathy, nephropathy, neuropathy) and cardiovascular risk factors is reinforced, with timelines unchanged from prior standards but with greater emphasis on earlier intervention when SGLT2 or GLP‑1 therapy is indicated.
Expert Commentary and Insights
Committee perspectives
– The ADA writing group emphasizes a shift from a glucose‑centric to a patient‑centered, cardiometabolic approach. That means when ASCVD, HF, or CKD is present—or at high risk—clinicians should prioritize agents that reduce those risks, sometimes regardless of baseline HbA1c.
Controversies and open questions
– Long‑term outcomes of dual incretin agents: although tirzepatide produces dramatic weight loss and glucose lowering, definitive cardiovascular outcome trials are pending; experts recommend using these agents mindful of potential benefits and unanswered long‑term safety questions.
– Cost and access: the writing group acknowledges serious disparities in access to GLP‑1s, dual agonists, SGLT2s, and CGM—costs and insurance barriers limit implementation of the Standards for many patients.
– Weight maintenance after drug discontinuation: the committee highlights that weight regain is common if AOMs are stopped; long‑term strategies and payer policies influence duration of therapy.
Future trends and research needs
– Cardiovascular outcome data for newer agents (tirzepatide and successors), longer‑term kidney and safety data, head‑to‑head trials comparing combination strategies, and pragmatic studies on implementation and equity are high priorities.
Practical Implications for Clinicians
– Be proactive about screening—screen adults ≥35 years and those with risk factors earlier.
– In patients with ASCVD, HF, or CKD, prioritize SGLT2 inhibitors or GLP‑1 receptor agonists with proven outcome benefits; discuss benefits independent of glucose lowering.
– Offer weight‑management discussion routinely. For many patients with T2D and obesity, AOMs (GLP‑1 or dual agonists) should be part of shared decision‑making.
– Expand sensible use of CGM to guide therapy, reduce hypoglycemia, and improve time‑in‑range metrics—especially for insulin users.
– Address access and affordability early—prior authorization, manufacturer assistance programs, and multidisciplinary care (diabetes education, nutrition) will be crucial to implementing these recommendations.
Patient vignette (illustrative)
Michael is a 52‑year‑old man with newly diagnosed T2D (A1c 8.3%), BMI 37 kg/m2, and a prior myocardial infarction. How to apply ADA 2024?
– Screening/diagnosis: diagnosis confirmed by HbA1c and elevated fasting glucose.
– Initial therapy: lifestyle plus metformin (if tolerated) and prompt addition of a GLP‑1 agonist with proven cardiovascular benefit (e.g., semaglutide or liraglutide) given his ASCVD to reduce future cardiovascular events and promote weight loss (Grade A).
– Consider SGLT2 inhibitor: if he also has CKD or heart failure or high risk, add an SGLT2 inhibitor for cardiorenal protection; monitor kidney function and educate on side effects.
– Technology: consider CGM to track time‑in‑range and guide medication titration and hypoglycemia prevention (if on insulin later).
– Counseling: discuss benefits, side effects (gastrointestinal effects, cost), and realistic expectations about weight loss and medication duration.
References
– American Diabetes Association. Standards of Care in Diabetes—2024. Diabetes Care. 2024;47(Suppl 1).
– Zinman B, et al. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N Engl J Med. 2015;373:2117–2128.
– Heerspink HJL, et al. Dapagliflozin in Patients with Chronic Kidney Disease. N Engl J Med. 2020;383:1436–1446.
– Marso SP, et al. Liraglutide and Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med. 2016;375:311–322.
– Gerstein HC, et al. Dulaglutide and Cardiovascular Outcomes in Type 2 Diabetes (REWIND). Lancet. 2019;394:121–130.
– US Food and Drug Administration. Mounjaro (tirzepatide) prescribing information. 2022. https://www.fda.gov
– US Food and Drug Administration. Zepbound (tirzepatide) approval (weight management). 2023. https://www.fda.gov
– U.S. Centers for Disease Control and Prevention. National Diabetes Statistics Report. 2022. https://www.cdc.gov/diabetes
Category: Clinical Updates