Highlight
– Identification of AH001 as a novel inhibitor of RhoA signaling targeting TRPV4-RhoA-RhoGDI1 axis.
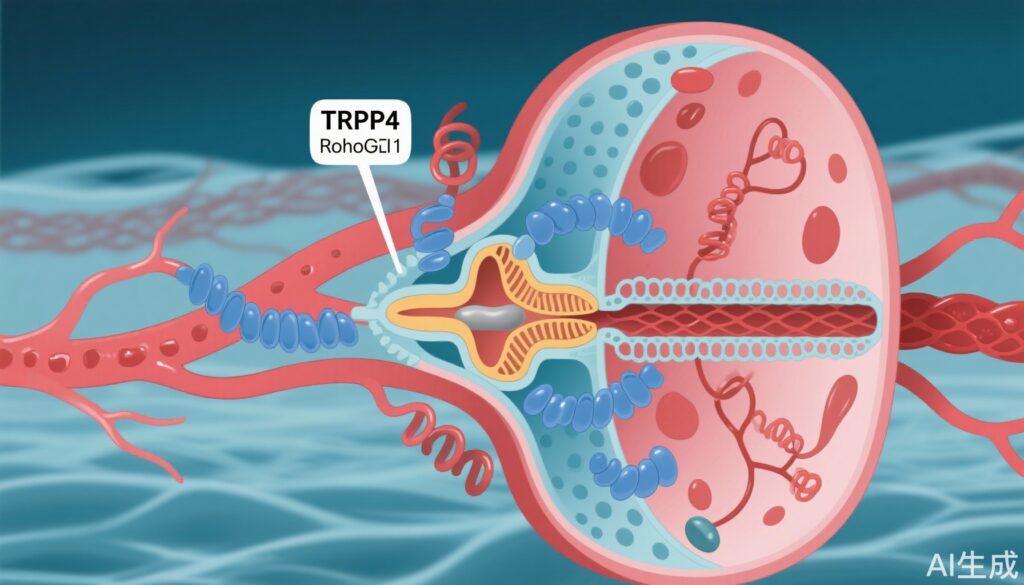
– Cryo-electron microscopy reveals AH001-bound TRPV4 traps RhoA in an inactive GDP-bound state.
– AH001 reduces blood pressure and prevents vascular remodeling in hypertensive animal models.
– The antihypertensive effects depend critically on TRPV4 and RhoGDI1 expression in vascular smooth muscle cells.
Study Background and Disease Burden
Hypertension remains a global public health challenge affecting over one billion individuals and constitutes a principal risk factor for cardiovascular disease, stroke, and kidney failure. Despite the availability of multiple antihypertensive drug classes, a significant portion of patients experience inadequate blood pressure control or adverse effects. The vascular smooth muscle cells (VSMCs) play a crucial role in determining vascular tone and resistance, directly influencing systemic blood pressure. The RhoA signaling pathway, mediated by the Rho family of small GTPases, is integral to VSMC contraction, cytoskeletal dynamics, and phenotype modulation. Aberrant RhoA activation contributes to sustained vasoconstriction and vascular remodeling in hypertension, making it an attractive therapeutic target. However, current RhoA pathway inhibitors face challenges including limited selectivity, low efficacy, and poor drug development feasibility. Therefore, novel strategies to inhibit RhoA signaling more specifically and effectively are urgently needed to improve hypertension treatment outcomes.
Study Design
This investigative study combined structural biology, molecular biology, cellular assays, and animal models to elucidate a novel regulatory axis for RhoA inactivation and its implications for hypertension therapy. Cryo-electron microscopy (cryo-EM), proximity ligation assay (PLA), and site-directed mutagenesis were employed to elucidate the interactions among TRPV4, RhoA, and RhoGDI1 proteins and the regulatory effects of the small molecule AH001. Primary VSMCs cultured from murine models, along with genetically engineered mice including Trpv4 knockout (Trpv4-/-) and smooth muscle-specific RhoGDI1 knockout (Arhgdiaf/f Myh11-CREERT2), were utilized to evaluate the molecular and physiological responses. Hypertensive models, including angiotensin II (Ang II)-induced hypertension and spontaneously hypertensive rats (SHR), served as in vivo platforms to test AH001’s efficacy. Key experimental endpoints included RhoA activity levels, VSMC contraction and phenotypic switching, blood pressure measurements, and vascular remodeling assessments.
Key Findings
The study identified AH001 ((R)-1-(3-ethylphenyl) ethane-1,2-diol) as a distinctive RhoA signaling pathway inhibitor operating via a unique mechanism. Unlike conventional RhoA inhibitors that often target RhoA directly or its downstream effectors, AH001 modulates the TRPV4-RhoA-RhoGDI1 axis to sequester the inactive RhoA-GDP form in the plasma membrane and cytoplasm.
Molecular and Structural Insights: Cryo-EM revealed that AH001 binds to TRPV4, stabilizing it in a closed conformation. This conformation enhances TRPV4’s affinity for RhoA, which remains in its inactive GDP-bound state, facilitating its association with RhoGDI1. This tripartite complex effectively reduces the pool of active, GTP-bound RhoA, thereby dampening downstream signaling. Site-directed mutagenesis confirmed key residues responsible for AH001-enhanced TRPV4-RhoA interaction. PLA assays further validated increased proximity among TRPV4, RhoA, and RhoGDI1 under AH001 treatment.
Cellular Functional Effects: AH001 treatment in cultured VSMCs resulted in substantial decreases in RhoA activity and concomitant inhibition of the RhoA/ROCK/MYPT1/MLC signaling pathway. Functionally, this translated into reduced VSMC contraction, a critical determinant of vascular tone. Additionally, AH001 suppressed the pathological phenotypic switch of VSMCs toward myofibroblast-like cells, which are implicated in vascular fibrosis and remodeling, by modulating the RhoA/ROCK/LIMK1/cofilin/MRTF-A/SRF signaling cascade. Knockdown of TRPV4 or RhoGDI1 markedly attenuated AH001’s inhibitory effects, underscoring the essential role of this axis in mediating AH001’s action.
In Vivo Antihypertensive Efficacy: In angiotensin II-induced hypertensive mice and spontaneously hypertensive rats, AH001 administration significantly lowered both acute and chronic blood pressure. It effectively prevented vascular remodeling, demonstrating protective vascular effects beyond blood pressure reduction. Notably, these beneficial effects were compromised in Trpv4-/- and Arhgdiaf/f Myh11-CREERT2 mice, confirming the necessity of intact TRPV4 and RhoGDI1 expression in VSMCs for AH001’s efficacy.
Taken together, AH001 represents a first-in-class molecule exploiting the TRPV4-RhoA-RhoGDI1 axis to modulate RhoA activity with superior specificity and functional impact compared to traditional approaches.
Expert Commentary
The discovery of AH001’s mechanism adds a novel dimension to targeting Rho GTPases, historically considered difficult to drug due to their high affinity for GTP/GDP and lack of obvious druggable pockets. By engaging TRPV4, a cation channel with known pharmacological tractability, this strategy employs an indirect yet effective route to sequester inactive RhoA. This approach may overcome longstanding issues of specificity and off-target effects encountered with direct RhoA or ROCK inhibitors.
Furthermore, the dual action of AH001 in both suppressing VSMC contraction and inhibiting maladaptive phenotypic switching holds promise for not only controlling hypertension but also mitigating vascular complications such as fibrosis and remodeling. This could translate into improved long-term cardiovascular outcomes.
Limitations include that AH001’s safety profile and pharmacokinetics in humans remain unexplored, and whether long-term modulation of TRPV4 impacts other physiological systems requires assessment. Also, clinical trial data are necessary to confirm translatability. Nonetheless, the mechanistic clarity and robust preclinical efficacy merit further development.
Conclusion
This study elucidates a previously unrecognized regulatory mechanism of RhoA signaling via the TRPV4-RhoA-RhoGDI1 axis and introduces AH001 as a pioneering inhibitor exploiting this pathway. The novel strategy of stabilizing inactive RhoA through TRPV4 modulation offers a promising therapeutic avenue for hypertension management with potential to improve specificity and minimize off-target effects. Future clinical evaluation is warranted to determine the safety and efficacy of AH001 or related compounds in hypertensive patients. More broadly, the findings open new research directions for targeting Rho family GTPases in vascular biology and beyond.
References
Wang J, Yuan Z, Yu N, Jiao Q, Zhou H, Liao W, Shan J, Ruan S, Zhao Y, Mo Y, Qi L, Li T, Fu J, Ke B, Xu Y, Qian X, Zhang J, Zhao Z, Li S, Wang R, Li H. Inactivation of RhoA for Hypertension Treatment Through the TRPV4-RhoA-RhoGDI1 Axis. Circulation. 2025 Aug 26;152(8):519-536. doi: 10.1161/CIRCULATIONAHA.124.071884. Epub 2025 Jun 16. PMID: 40518994.