Highlight
– Aberrant activation of the Hippo/YAP signaling pathway is critical in hepatocellular carcinoma (HCC) development.
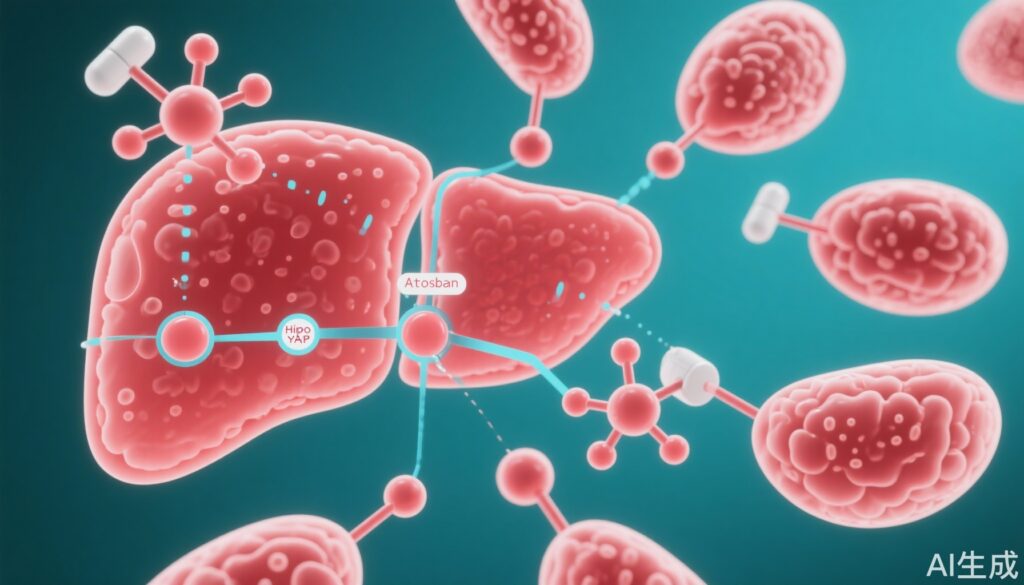
– The oxytocin receptor (OXTR), a G protein-coupled receptor, activates Hippo/YAP signaling via Gq/11 and fosters a positive feedback loop facilitating liver cancer progression.
– Atosiban, an OXTR antagonist and established tocolytic agent, effectively disrupts this positive feedback, suppressing tumor growth in multiple preclinical HCC models.
– This discovery opens an avenue to repurpose Atosiban and highlights OXTR as a promising therapeutic target in HCC, addressing an unmet clinical need for effective treatment strategies.
Study Background and Disease Burden
Hepatocellular carcinoma (HCC) represents the most common form of primary liver cancer globally, ranking as a leading cause of cancer-related mortality. Despite advances, prognosis remains poor, particularly in advanced stages due to limited effective therapeutic options. Molecularly, aberrant activation of the Hippo signaling pathway effector Yes-associated protein (YAP) is strongly implicated in HCC pathogenesis, promoting oncogenic transcriptional programs and tumor progression.
Efforts to directly inhibit YAP have encountered setbacks: notably, verteporfin, a YAP inhibitor, failed to demonstrate efficacy in clinical trials potentially due to drug instability and off-target toxicity. Consequently, research focus has shifted towards upstream regulators such as G protein-coupled receptors (GPCRs), which modulate Hippo/YAP signaling. Given the vast diversity of GPCRs and their varying G protein partners, pinpointing the key receptor driving HCC remained challenging.
Study Design
A collaborative Chinese research team from China Medical University, Henan University of Chinese Medicine, and Shandong University conducted an extensive screening of FDA-approved drugs targeting GPCRs to identify candidates affecting Hippo/YAP activity. They used a combined approach involving gene set enrichment analysis (GSEA) to correlate GPCR expression with Hippo/YAP pathway activation, followed by an siRNA functional screen in liver cancer cell lines and cross-referencing to 134 FDA-approved GPCR-targeting drugs.
The oxytocin receptor (OXTR) emerged as a top candidate. Researchers then evaluated OXTR expression levels in HCC vs. normal liver tissues and assessed clinical correlations with patient outcomes. Functional studies involved OXTR knockdown or overexpression in vitro and in vivo using multiple HCC mouse models: subcutaneous and orthotopic xenografts, patient-derived organoids, and genetically engineered MST1/2 double knockout mice. Mechanistic investigations explored OXTR interactions with Gq/11 proteins, downstream Rho/ROCK pathways, LATS1 kinase activity, and YAP phosphorylation status. Chromatin immunoprecipitation (ChIP) assays elucidated the feedback regulation between YAP and OXTR transcription. Finally, the impact of Atosiban, an OXTR antagonist approved for early labor treatment, on tumor growth and signaling was evaluated in these models.
Key Findings
Identification of OXTR as a Key Regulatory GPCR in HCC
OXTR expression was significantly upregulated at both mRNA and protein levels in HCC tissues compared to adjacent normal liver. High OXTR expression strongly correlated with worse overall survival and prognosis in HCC patients. Functional silencing of OXTR suppressed HCC cell proliferation and reduced tumor burden in mouse models, whereas OXTR overexpression had the opposite effect, confirming its tumor-promoting role.
Mechanistic Insights: OXTR-Gq/11-Hippo/YAP Axis Activation
OXTR activates the Hippo/YAP pathway via coupling with the Gq/11 subunit, subsequently stimulating the Rho/ROCK signaling cascade. This activation leads to inhibition of LATS1 kinase, a core Hippo pathway component responsible for YAP phosphorylation. Reduced LATS1 activity results in dephosphorylation and nuclear accumulation of YAP, which forms active TEAD transcriptional complexes promoting oncogenic gene expression driving HCC progression.
Moreover, ChIP assays demonstrated that activated YAP binds to enhancer regions of the OXTR gene itself, enhancing OXTR transcription. This establishes a positive feedback loop that further sustains Hippo/YAP signaling and tumor growth.
Therapeutic Potential of Atosiban
Atosiban, a competitive OXTR antagonist currently used clinically to prevent preterm labor, was shown to disrupt the OXTR-YAP positive feedback loop, demonstrated by increased YAP phosphorylation (inactive form) and suppression of downstream oncogenic transcription.
In multiple HCC mouse models, including xenograft and genetically engineered variants, Atosiban treatment markedly inhibited tumor growth and reduced cancer cell proliferation. This result highlights the potential utility of repurposing Atosiban as a novel therapeutic agent targeting OXTR in liver cancer.
Expert Commentary
This study provides a compelling mechanistic framework integrating GPCR signaling with Hippo/YAP pathway dysregulation in HCC. The identification of OXTR as a driver of tumor progression and its modulation by a clinically approved drug is an exciting advance poised to impact translational cancer therapeutics.
While verteporfin’s failure in clinical trials dampened YAP-targeted therapy prospects, targeting upstream GPCRs offers a viable alternative with potentially fewer off-target effects. The repurposing of Atosiban is especially promising given its established safety profile in pregnancy. However, clinical validation is required to confirm effectiveness and safety in HCC patients.
Limitations include the predominantly preclinical nature of current evidence and the need to understand potential heterogeneity in OXTR expression across patient subgroups. Additionally, combinatorial approaches incorporating Atosiban with existing HCC treatments merit investigation to maximize therapeutic benefit.
Conclusion
In summary, this pioneering work identifies the oxytocin receptor as a critical upstream regulator of the Hippo/YAP signaling pathway that drives hepatocarcinogenesis. The FDA-approved drug Atosiban effectively blocks this receptor, disrupts a positive oncogenic feedback loop, and inhibits HCC growth in relevant preclinical models. These findings provide a novel molecular target and repurposable therapeutic approach for liver cancer, addressing an urgent clinical need. Further clinical studies should assess Atosiban’s efficacy and integration into HCC treatment paradigms.
References
1. Yang H, Cui J, Su P, et al. Oxytocin Receptor Regulates the Hippo/YAP Axis to Drive Hepatocarcinogenesis. Cancer Res. Published online July 31, 2025. doi:10.1158/0008-5472.CAN-24-3405